2154
Validating UTE-based Assessment of Bronchopulmonary Dysplasia at 3T
Narayan Iyer1,2, Matthew Borzage1,2, and Eamon Doyle2,3
1Pediatrics, Children's Hospital of Los Angeles, Los Angeles, CA, United States, 2Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 3Radiology, Children's Hospital of Los Angeles, Los Angeles, CA, United States
1Pediatrics, Children's Hospital of Los Angeles, Los Angeles, CA, United States, 2Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 3Radiology, Children's Hospital of Los Angeles, Los Angeles, CA, United States
Synopsis
Keywords: Lung, Pediatric, BPD, UTE
Bronchopulmonary dysplasia (BPD) is the most common serious complication of prematurity affecting approximately 30,000 infants annually in the USA alone. Decisions regarding treatment course as well as prognostication require an understanding of regional lung changes. However, chest radiographs poorly distinguish the severity of BPD. Advances in ultra-short echo time imaging have made lung imaging feasible at clinically relevant field strengths. We have implemented a UTE-based imaging protocol at our institution and started validation in pre-term infants. Initial results demonstrate that clinically interpretable images can be acquired in an infant with BPD.Introduction
Bronchopulmonary dysplasia (BPD) is the most common serious complication of prematurity affecting approximately 30,000 infants annually in the USA alone1,2. Despite advances in neonatal care, two thirds of infants with BPD have severe disease leading to an estimated 1,000 to 2,400 deaths annually3,4. Lack of a safe and reliable tool to assess severity of ongoing lung disease has prevented the development of accurate disease classification and slowed the development and testing of effective therapies. Pulmonary function testing is highly specialized in children younger than 4 years age which has led to poor understanding of the post-neonatal course of BPD. The current definition of BPD does not discriminate the categories of severity sufficiently to predict respiratory outcomes beyond the first5. Decisions about the need and timing of tracheostomy and need for home oxygen are just a few examples of how a diagnostic tool that could measure the severity of BPD accurately could significantly impact the ongoing care of BPD infants in the newborn intensive care unit (NICU). Chest radiographs poorly distinguish the severity of BPD6. Chest CT scan can inform immediate management of infants with BPD in the NICU and provide prognostication7,8. However, exposure risks from ionizing radiation are increased in pediatric populations and increase with follow up studies9,10. MRI offers a tool that does not expose infants to ionizing radiation. However, there are several problems associated with using MRI to image lung parenchyma and airways. The proton density of lung parenchyma is about 20% that of the surrounding muscle and as a result the parenchymal signal intensity is reduced compared to chest wall and mediastinal soft tissue. The air-tissue interface in the lung contributes to magnetic inhomogeneity and rapid signal decay. Finally, respiratory and cardiac motion degrades image quality. Newer MRI technologies that use sub-millisecond echo times have been proposed to capture lung parenchymal signals before it decays11-15. Studies using ultra-short echo times (UTE) on 1.5 Tesla (T) MRI machines have been shown to distinguish between areas with different parenchymal densities and radiologic severity has been associated with clinical severity of BPD16-18. Several barriers remain before MRI can become a widely used biomarker for BPD.Methods
MR imaging was performed on a Philips Ingenia (Best, Netherlands) 3T magnet with a 70cm bore with IRB approval (CCI# CHLA-21-00081). During a clinically indicated brain MRI, an additional lung imaging sequence was performed with a center-out radial stack-of-stars sequence with the following sequence parameters: TE = 0.18ms, TR=3.29ms, pixel size=1.19x1.19mm2, matrix=268x268, slice thickness=4mm, slice gap=4mm, bandwidth = 1412 Hz/px, NSA=1. Whole lung coverage was specified. A respiratory navigator was placed on the dome of the liver. Chest x-ray was performed clinically.Results
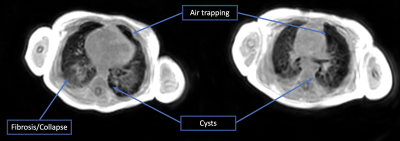
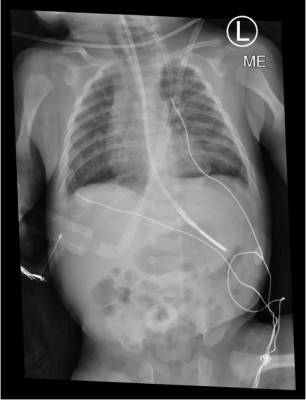
MR examination was performed on a single 4 month old (46 weeks corrected gestational age) infant born prematurely at 28 weeks gestation (3 months preterm) with a birth weight of 1kg. Initially requiring non-invasive respiratory support after birth, the infant required endotracheal intubation and surfactant treatment. Subsequently, the infant required four weeks of invasive mechanical ventilation and a further 14 weeks of non-invasive respiratory support. Currently, the infant is on low flow oxygen with normal growth parameters. The MRI demonstrated bilateral cystic changes with areas of collapse and consolidation in posterior regions bilaterally (figure 1). The chest x-ray is very non-specific showing bilateral chronic infiltrates (figure 2).Discussion
Compared to the chest x-ray and the BPD classification system, the UTE-based MR images demonstrated changes that are much more reflective of the clinical course and current respiratory status. BPD and especially severe BPD is characterized by increased airway resistance and heterogenous lung disease. Severe heterogeneity produced by cystic changes in a lung with BPD can complicate respiratory management in the NICU. Identification of patients with a high burden of cystic lung tissue is clinically valuable as it could directly inform ventilatory strategy. Recently lung MRI has also been proposed as an objective tool to make judgements on the appropriateness of tracheostomy in infants with BPD19.There are significant challenges to lung MRI in infants. The low proton density in the lungs requires a variety of technical tradeoffs, such as reduced resolution to achieve sufficiently short echo times. Additionally, while adults can perform breath holds, infants cannot follow instructions and breath 2-4 times faster than adults. The increased respiratory rate and reduced diaphragm excursion can make respiratory navigation more challenging. Despite these challenges, UTE-based MRI was able to provide a clinically interpretable image in a free-breathing infant undergoing a routine brain MRI.
Conclusion
UTE imaging at 3T shows promise for the assessment and management of BPD in preterm infants. Imaging lung changes in BPD with MRI will likely allow for better disease categorization that more correctly reflects physiological derangements seen in infants with BPD. Because MRI does not involve radiation, repeated studies as part of longitudinal research protocols will allow for better elucidation of the natural history of BPD. The demonstration of successful imaging at our institution is an important step toward validation of techniques that have been described at other institutions. Additional subjects and further cross-vender and cross-institution validation is needed.Acknowledgements
The authors thank Jason Woods for his assistance defining and implementing the imaging protocol used in this work.References
- Lapcharoensap W, Gage SC, Kan P, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr 2015;169:e143676.
- Martin JA, Osterman MJK. Describing the Increase in Preterm Births in the United States, 2014-2016. NCHS Data Brief 2018:1-8.
- Murthy K, Porta NFM, Lagatta JM, et al. Inter-center variation in death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol 2017;37:723-7.
- Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011;31:635-40.
- Poindexter BB, Feng R, Schmidt B, et al. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc 2015;12:1822-30.
- Northway WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357-68.
- Wu KY, Jensen EA, White AM, et al. Characterization of Disease Phenotype in Very Preterm Infants with Severe Bronchopulmonary Dysplasia. American journal of respiratory and critical care medicine 2020;201:1398-406.
- van Mastrigt E, Logie K, Ciet P, et al. Lung CT imaging in patients with bronchopulmonary dysplasia: A systematic review. Pediatr Pulmonol 2016;51:975-86.
- Goodman TR, Mustafa A, Rowe E. Pediatric CT radiation exposure: where we were, and where we are now. Pediatr Radiol 2019;49:469-78.
- Kino A, Zucker EJ, Honkanen A, et al. Ultrafast pediatric chest computed tomography: comparison of free-breathing vs. breath-hold imaging with and without anesthesia in young children. Pediatr Radiol 2019;49:301-7.
- Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 2013;70:1241-50.
- Lederlin M, Crémillieux Y. Three-dimensional assessment of lung tissue density using a clinical ultrashort echo time at 3 tesla: a feasibility study in healthy subjects. J Magn Reson Imaging 2014;40:839-47.
- Bell D, Wright G. A retrospective review of the palliative surgical management of malignant pleural effusions. BMJ Support Palliat Care 2013.
- Triphan SM, Jobst BJ, Breuer FA, et al. Echo time dependence of observed T1 in the human lung. J Magn Reson Imaging 2015;42:610-6.
- Triphan SM, Breuer FA, Gensler D, Kauczor HU, Jakob PM. Oxygen enhanced lung MRI by simultaneous measurement of T1 and T2 * during free breathing using ultrashort TE. J Magn Reson Imaging 2015;41:1708-14.
- Higano NS, Spielberg DR, Fleck RJ, et al. Neonatal Pulmonary Magnetic Resonance Imaging of Bronchopulmonary Dysplasia Predicts Short-Term Clinical Outcomes. American journal of respiratory and critical care medicine 2018;198:1302-11.
- Higano NS, Adaikalam S, Hysinger EB, et al. Clinically-relevant tracheostomy prediction model in neonatal bronchopulmonary dysplasia via respiratory MRI. European Respiratory Journal 2020;56:4789.
- Ochiai M, Hikino S, Yabuuchi H, et al. A new scoring system for computed tomography of the chest for assessing the clinical status of bronchopulmonary dysplasia. J Pediatr 2008;152:90-5, 5.e1-3.
- Hahn AD, Higano NS, Walkup LL, et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging 2017;45:463-71.
DOI: https://doi.org/10.58530/2023/2154