2135
Self-Navigated Rapid Radial Free-Breathing Liver MR Elastography: Assessment of Technical Performance in Children at 3T
Sevgi Gokce Kafali1,2, Bradley D. Bolster Jr.3, Shu-Fu Shih1,2, Timoteo I. Delgado1,4, Xiaodong Zhong5, Vibhas Deshpande6, Timothy R. Adamos7, Shahnaz Ghahremani1,7, Kara L. Calkins7, and Holden H. Wu1,2,4
1Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 2Bioengineering, Henry Samueli School of Engineering, University of California, Los Angeles, Los Angeles, CA, United States, 3US MR R&D Collaborations, Siemens Medical Solutions USA, Inc., Salt Lake City, UT, United States, 4Physics and Biology in Medicine Interdepartmental Program, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 5US MR Collaborations, Siemens Medical Solutions USA, Inc., Los Angeles, CA, United States, 6US MR Collaborations, Siemens Medical Solutions USA, Inc., Austin, TX, United States, 7Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
1Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 2Bioengineering, Henry Samueli School of Engineering, University of California, Los Angeles, Los Angeles, CA, United States, 3US MR R&D Collaborations, Siemens Medical Solutions USA, Inc., Salt Lake City, UT, United States, 4Physics and Biology in Medicine Interdepartmental Program, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 5US MR Collaborations, Siemens Medical Solutions USA, Inc., Los Angeles, CA, United States, 6US MR Collaborations, Siemens Medical Solutions USA, Inc., Austin, TX, United States, 7Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Keywords: Elastography, Liver
Hepatic stiffness measured by MR elastography (MRE) is a biomarker for fibrosis. Standard liver MRE requires breath-holding (BH) to avoid motion artifacts, but BH is challenging for children. Radial free-breathing (FB) MRE can overcome this challenge but requires longer scan times. Radial FB-MRE using rapid wave encoding decreases the scan time and self-navigation supports motion compensation. With the help of fractional encoding to reduce echo times, image quality can be further improved. This work demonstrated that self-navigated rapid and rapid fractional FB-MRE methods measure hepatic stiffness with close agreement and similar repeatability compared to corresponding BH-MRE in children at 3T.Introduction
Hepatic stiffness (HS) measured by magnetic resonance elastography (MRE) is a biomarker to detect hepatic fibrosis 1-4. Standard Cartesian liver MRE requires breath-holding (BH) to mitigate motion artifacts, which is challenging for children 5. Radial sampling is motion-robust and provides self-navigation signals from the center of k-space for motion compensation (e.g., reject/accept data acquired out/inside of a desired range of motion) 6,7. Previously, radial free-breathing (FB) gradient-echo (GRE) MRE of the liver demonstrated agreement with BH Cartesian GRE MRE 8. However, radial FB-MRE required several minutes to acquire each slice 8. Rapid wave encoding can cut the scan duration of GRE-based MRE in half 9. This is especially beneficial for radial FB-MRE since oversampled acquisition is preferred to reduce undersampling artifacts after self-navigated motion compensation 10-12. Additionally, fractional encoding decreases echo time (TE) and can improve image quality especially in regions with short T2* (e.g., due to liver iron content) 13, 14. Our goal is to perform a systematic technical assessment of self-navigated rapid (r) and rapid fractional (rfr) radial FB-MRE for measuring HS, in terms of agreement and repeatability with respect to their Cartesian BH-MRE counterparts, in children at 3T.Methods
Study Cohort and MRE Experiments:This IRB-approved HIPAA-compliant study enrolled 30 children, including 21 healthy children (10F, 11M) and 9 overweight children (body mass index [BMI] >85th percentile; 3F, 6M). The [median, interquartile range (IQR)] age was [13.5, (11.9, 14.9)] years and BMI was [19.0, (17.1, 22.1)] kg/m2 with [51st, (18th, 92nd)] percentile. For repeatability, all four sequence variants (rBH-MRE, rfrBH-MRE, research applications rFB-MRE, and rfrFB-MRE) were acquired twice (scan 1 and 2) in the same exam on a 3T scanner (MAGNETOM PrismaFit, Siemens Healthcare GmbH, Erlangen, Germany). The imaging parameters were matched as much as possible (Table 1). Due to time constraints, 2 slices (matched to BH-MRE slices) were acquired for FB-MRE sequences, which were single-slice acquisitions. A prototype flexible MRE passive driver (Mayo Clinic, Rochester, MN) was used to improve subject comfort.
MRE Reconstruction:
rFB-MRE and rfrFB-MRE employed self-navigated motion compensation with 60% acceptance rate (Fig. 1). To reduce undersampling artifacts after self-navigated motion compensation, FB-MRE sequences were oversampled by a factor of 1.5x (net undersampling factor of 0.9). All BH- and FB-MRE images were reconstructed inline on the scanner. As in 5,8, HS was measured inside regions of interest (ROI) in the liver with 90% confidence interval (CI) (Fig. 1C).
Analysis:
Bland-Altman (BA) analysis of the mean differences (MD) and 95% limits of agreements (LoA) was used to evaluate the agreement between matched methods: 1. rBH-MRE and rFB-MRE, 2. rfrBH-MRE and rfrFB-MRE 15,16. Repeatability was assessed using BA analysis and the within-subject coefficient of variation (wCV), as recommended by the Quantitative Imaging Biomarkers Alliance (QIBA) profile for MRE of the liver 17.
Results
Figure 2 shows representative magnitude images, wave images, and HS maps. All four MRE sequences yielded similar HS for the chosen slice. rBH-MRE and rFB-MRE agreed with MD of 0.05 kPa and 0.09 kPa for scan 1 and 2, respectively (Figure 3). For rfrBH-MRE versus rfrFB-MRE, the MD in scans 1 and 2 were 0.12 kPa and 0.09 kPa, respectively. All four sequences were repeatable with MD ~0 kPa and wCV of 5.3% and 6.3% for rBH-MRE and rFB-MRE, and 4.3% and 3.1% for rfrBH-MRE and rfrFB-MRE (Figure 4).Discussion
We evaluated the technical performance of self-navigated rapid and rapid-fractional radial FB-MRE for measuring HS in children at 3T. Corresponding FB-MRE and BH-MRE methods agreed with small MD. All MRE techniques achieved wCV<7%, indicating acceptable repeatability according to QIBA 17. rfrFB-MRE was the most repeatable sequence with smallest wCV and LoA.For comparison, we matched the reconstructed in-plane resolution for FB-MRE and BH-MRE. As the FB-MRE sequences did not yet perform spatial interpolation, this required higher acquired in-plane resolution and longer scan time. Future development of FB-MRE can implement interpolation to allow reduction of the acquired resolution. With this, the FB-MRE scan time can decrease to ~1min/slice.
The quality of the FB-MRE magnitude images was often improved with fractional encoding (vs. no fractional encoding) (Figure 2). FB imaging with uncompensated or residual motion effects might increase the apparent R2* and lead to faster signal decay 18. Therefore, the combination of fractional encoding (to reduce TE) and self-navigation for FB-MRE might benefit the image quality and quantification accuracy in liver tissue experiencing motion, especially when there is higher iron content.
While this study included one of the largest cohorts of children for radial FB-MRE, the subjects in the current cohort had a limited range of HS and R2* ([24.4, 84.8] s-1). As only one subject had liver R2*>60 s-1 (upper bound of normal range19), the benefits of fractional encoding might not be adequately characterized. Future work can include more subjects with a wider range of HS and R2* values to evaluate FB-MRE.
Conclusion
Self-navigated rapid and rapid fractional radial FB-MRE methods measured hepatic stiffness with close agreement and similar repeatability compared to corresponding Cartesian BH-MRE in children at 3T. Radial FB-MRE techniques could be promising for pediatric imaging when breath-holding is a challenge.Acknowledgements
This work was supported by an Exploratory Research Grant from the UCLA Department of Radiological Sciences, the National Institutes of Health under Award Numbers NIH/NIDDK R01DK124417 and NIH/NIBIB U01EB031894, and the National Center for Advancing Translational Sciences under Award Number UL1TR001881.The authors thank investigators at the Mayo Clinic for providing the prototype flexible MRE passive driver suitable for children. The authors thank the clinicians, study coordinators and the MRI technologists at UCLA.References
1. Idilman IS, Li J, Yin M, Venkatesh SK. MR elastography of liver: current status and future perspectives. Abdom Radiol 2020:1-19.2. Batheja M, Vargas H, Silva AM, et al. Magnetic resonance elastography (MRE) in assessing hepatic fibrosis: performance in a cohort of patients with histological data. Abdom Imaging 2015;40(4):760-765.
3. Xanthakos SA, Podberesky DJ, Serai SD, et al. Use of Magnetic Resonance Elastography to Assess Hepatic Fibrosis in Children with Chronic Liver Disease. J Pediatr-Us 2014;164(1):186-188.
4. Singh S, Venkatesh S, Wang Z, et al. Diagnostic Performance of Magnetic Resonance Elastography for the Staging of Liver Fibrosis: A Systematic Review and Collaborative Individual Participant Data Meta-Analysis. Am J Gastroenterol 2014;109:S144-S144.
5. Schwimmer JB, Behling C, Angeles JE, et al. Magnetic Resonance Elastography Measured Shear Stiffness as a Biomarker of Fibrosis in Pediatric Nonalcoholic Fatty Liver Disease. Hepatology 2017;66(5):1474-1485.
6. Kafali SG, Armstrong T, Shih S-F, et al. Assessment of Free-Breathing Radial Magnetic Resonance Elastography in Healthy Children and Children with Liver Disease at 3T. 28th Annual Meeting of International Society of Magnetic Resonance in Medicine; 2020. p. Program Number 0087.
7. Holtrop JL, Kannengiesser S, Loeffler RB, Song R, Hillenbrand CM. Free Breathing Radial Magnetic Resonance Elastography. 27th Annual Meeting of International Society of Magnetic Resonance in Medicine. Montreal, Canada; 2019. p. Program Number 4482.
8. Kafali SG, Armstrong T, Shih S-F, et al. Free-breathing radial magnetic resonance elastography of the liver in children at 3 T: a pilot study. Pediatric Radiology 2022:1-12.
9. Chamarthi SK, Raterman B, Mazumder R, et al. Rapid acquisition technique for MR elastography of the liver. Magn Reson Imaging 2014;32(6):679-683.
10. Kafali SG, Bolster BD, Shih S-F, et al. Radial Free-Breathing Liver MR Elastography in Children using Self-Navigation and Rapid Fractional Encoding. Magnetic Resonance Elastography Workshop of International Society of Magnetic Resonance in Medicine. Berlin, Germany; 2022.
11. Kafali SG, Bolster BD, Shih S-F, et al. Self-Navigated Radial Free-Breathing Magnetic Resonance Elastography of the Liver with Rapid Motion Encoding in Children at 3T. 30th Annual Meeting of International Society of Magnetic Resonance in Medicine. London, UK; 2022.
12. Zhong X, Hu HH, Armstrong T, et al. Free‐Breathing Volumetric Liver and Proton Density Fat Fraction Quantification in Pediatric Patients Using Stack‐of‐Radial MRI With Self‐Gating Motion Compensation. Journal of Magnetic Resonance Imaging 2021;53(1):118-129.
13. Rump J, Klatt D, Braun J, Warmuth C, Sack I. Fractional encoding of harmonic motions in MR elastography. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2007;57(2):388-395.
14. Felker ER, Choi KS, Sung K, et al. Liver MR Elastography at 3 T: Agreement Across Pulse Sequences and Effect of Liver R2*on Image Quality. Am J Roentgenol 2018;211(3):588-594.
15. Obuchowski NA, Reeves AP, Huang EP, et al. Quantitative imaging biomarkers: a review of statistical methods for computer algorithm comparisons. Statistical methods in medical research 2015;24(1):68-106.
16. Raunig DL, McShane LM, Pennello G, et al. Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Statistical methods in medical research 2015;24(1):27-67.
17. Committee QMB. MR Elastography of the Liver, Quantitative Imaging Biomarkers Alliance. Profile Stage: Consensus. June 6, 2019.
18. Zhong X, Armstrong T, Nickel MD, et al. Effect of respiratory motion on free‐breathing 3D stack‐of‐radial liver relaxometry and improved quantification accuracy using self‐gating. Magnetic Resonance in Medicine 2020;83(6):1964-1978.
19. Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. Journal of Magnetic Resonance Imaging 2007;25(3):540-547.
Figures
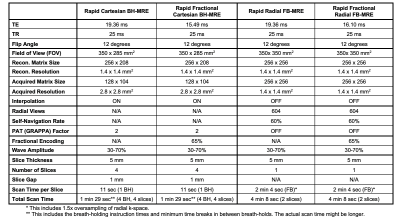
Table 1. Representative imaging parameters. The imaging parameters used for the Cartesian and radial MRE sequences were matched as much as possible. MRE wave amplitudes were set according to the body-mass-index of the subject and were fixed for all four sequences. MRE wave frequency was set to 60Hz. TE: echo time; TR: repetition time; BW: readout bandwidth; MEG: motion encoding gradients. PAT: parallel imaging. GRAPPA: Generalized Auto-calibrating Partial Parallel Acquisition. N/A: not applicable.
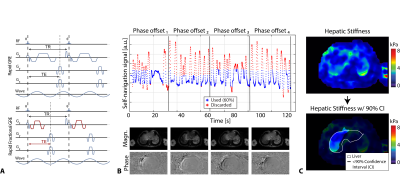
Figure 1. The image acquisition and processing pipeline for radial FB-MRE. A) Sequence diagrams for rapid and rapid fractional GRE-based radial FB-MRE. B) Data acquisition for 4 wave phase-offset images. Both FB-MRE variants were oversampled by a factor of 1.5x during acquisition. The self-navigation signal for a representative subject with an acceptance rate of 60% is shown. C) Using the stiffness maps from scanner software, hepatic stiffness was measured in regions of the liver (white contours) with $$$\geq$$$90% confidence interval (CI), at the regions outside the black masks.
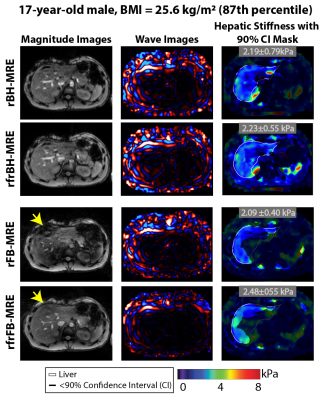
Figure 2. Representative magnitude images, wave images, 90% confidence interval (CI) masks, and liver contours (white lines) are shown for rapid (r) and rapid-fractional (rfr) Cartesian BH-MRE, and their free-breathing counterparts r and rfr radial FB-MRE. rfrFB-MRE improved the magnitude image quality when compared to rFB-MRE (yellow arrows). Mean hepatic stiffness values measured inside regions of the liver with $$$\geq$$$ 90% CI for the sequences were consistent with each other for this representative subject (17-year-old male with BMI around 87th percentile).
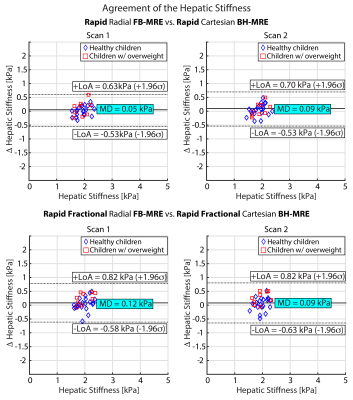
Figure 3. Agreement in hepatic stiffness (n=30) was assessed using Bland-Altman plots with 95% limits of agreement (LoA) and mean difference (MD). The MD and LoA for Cartesian BH-MRE and radial FB-MRE techniques show close agreement for the hepatic stiffness measurements. σ: standard deviation of the difference in hepatic stiffness.
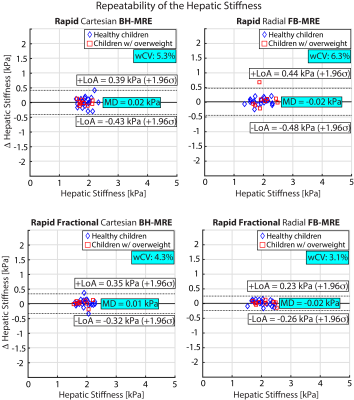
Figure 4. Repeatability of the hepatic stiffness values (n=30) in all tested MRE sequences was assessed by Bland-Altman plots with 95% limits of agreement (LoA) and mean difference (MD). The within-subject coefficient of variation (wCV) was also calculated. All four sequences produced repeatable hepatic stiffness measurements, with rapid fractional FB-MRE producing the smallest LoA as well as wCV. σ: standard deviation of the difference in hepatic stiffness of two repeated exams.
DOI: https://doi.org/10.58530/2023/2135