2133
Quantitative MRI for radiation oncology patients with head and neck squamous cell carcinoma1BWH, Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Head & Neck/ENT, Relaxometry, Radiotherapy, treatment, tumor
Radiation oncology patients often undergo longitudinal scans to evaluate the effect of treatments on lesions, and to monitor surrounding tissues. We are considering the inclusion of quantitative MRI in current imaging protocols. To this end, we implemented a simple quantitative imaging technique and tested it in a small cohort of five patients. Results captured differences in T1 and T2 between tissues on the treated side vs. the contralateral tissues. Little work has been done so far with MRI relaxometry for monitoring treatment in head and neck squamous cell carcinoma, and this work represents an early step in this direction.Introduction
In radiation oncology applications, quantitative MRI could supplement the qualitative grayscale images typically generated by MRI. Quantitative images have meaningful physical units and are mostly immune to biases caused by spatial variation in receive-coil sensitivity. Acquiring them at different time points during the course of a fractionated treatment could provide quantitative predictors of treatment response and predict adverse outcomes for surrounding tissues subjected to irradiation. For example, changes in T1 and T2 values have been observed for the parotid and submandibular glands after radiotherapy1, although correlation with xerostomia and periodontic-endodontic disease is not yet established.Standard MR relaxometry sequences necessitate multiple scans to produce either T1 or T2 maps and may be considered too time consuming for clinical implementation. Faster approaches such as Look-Locker2, and those calculating both T1 and T2 jointly such as SyMRI3-5, MR fingerprinting (MRF)6,7 or TESS8 are not readily available on most clinical scanners. To circumvent this problem we used a recently proposed method that can be implemented on most MRI scanners and that involves publicly available GitLab code for reconstruction9. This method, originally proposed for brain imaging, was employed here for the more challenging neck/skull-base, where air pockets, water- and fat-based tissues are closely intermixed. T1 and T2 maps, along with idealized proton-density weighted (PDW) images (‘idealized’ in the sense that they mathematically represent a case where TR tends toward infinity and TE toward zero) were jointly created. More generally, there has been limited use of MRI relaxometry in neck/skull-base radiation oncology10, and the present work represents an early step in this direction.
Methods
Five patients provided informed consent under an IRB-approved protocol. All had primary head and neck squamous cell carcinoma and were undergoing radiotherapy at our institution. Treatment immobilization masks are typically worn during imaging for positional reproducibility. Because such masks are generally too large for regular head coils, two UltraFlexLarge18 coils were instead wrapped around the head and mask11 and a Body18 coil covered the neck area (Fig. 1a). The quantitative imaging protocol9, based on a product multi-shot spin-echo EPI sequence available on our scanner (Siemens Vida 3T), was added to our regular protocol. The reconstruction code was available for download from GitLab. A main characteristic of the approach is the mathematical modeling of the signal and corresponding numerical solution, which allows T1, T2 and PDW images to be jointly reconstructed. Ten slices were mapped in less than five minutes of scan time (192×192 matrix, 240×240 mm2 FOV, 1.25×1.25×5 mm3 resolution, ETL of 11, water-only excitation). The PDW images, T1 and T2 maps were visually assessed and compared to the morphological sequences. Mean T1 and T2 values over ROIs were used for comparison with literature values and to compare tissues on treated and contralateral locations.Results
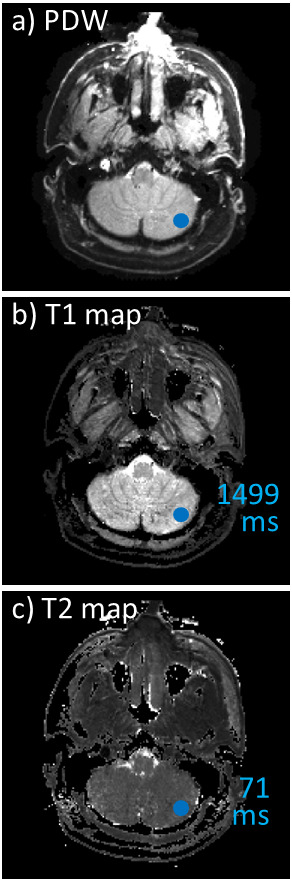
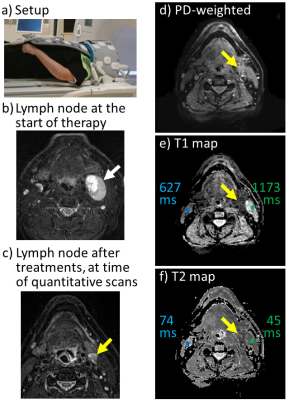
Similar image quality was obtained for all five recruited patients, and example images are shown in Fig. 1 (patient #3/4, slice #10/10) and Fig. 2 (patient #1/4, slice #5/10). In addition to our usual protocol, the quantitative imaging method provided idealized PDW images, T1 and T2 maps (Fig. 1,2). Images in Fig. 1 included cerebellum tissues, and mean T1 and T2 values were calculated for the ROI shown in blue. The results, 1499 ms for T1 and 71 ms for T2, appear reasonable for a heterogenous ROI expected to include a mixture of white matter (WM) and gray matter (GM)12; since slice coverage barely reached the cerebellum separate WM and GM ROIs could not readily be drawn.Figure 2b-f shows a malignant lymph node near the pharynx, along with surrounding tissues, for patient #1/4. More specifically, Fig. 2b was obtained at an earlier date and shows the lymph node before treatments (white arrow). In contrast, on the day that the patient was recruited and with treatment partly completed the lymph node was much reduced in size (Fig. 1c, yellow arrow). Slice #5 (out of 10) is shown in Fig. 1d-f; this slice captures the lesion and surrounding tissues. Mean T1 and T2 values are provided for small ROIs on the treated side (green ROI and numbers in Fig. 1e-f) as well as the contralateral side (blue ROI and numbers in Fig. 1e-f). Higher T1 and lower T2 values were observed on the treated side vs. the contralateral side.
Discussion
The current study demonstrated the feasibility of implementing a simple quantitative mapping method from the literature9 in a head/neck cancer application. Although the patient cohort was small, our results suggest that quantitative MRI helped capture differences in tissue properties between treated and contralateral sides. A longitudinal study, where patients are imaged with quantitative imaging at different time points during treatment, would help establish causality between dose and observed changes, and possibly help establish clinical utility.Conclusion
A quantitative MRI mapping approach was easily implemented on a 3T scanner employed for MR simulations (i.e., treatment planning). Given that ten slices were mapped in less than five minutes, these scans could readily be included in a clinical protocol. Results suggested that radiation-induced tissue changes might be captured by the approach.Acknowledgements
Support from NIH grant R01EB030470, and from a Kayes technology grant 2019, is duly acknowledged.References
1. Stieb S, Elgohari B, Fuller CD. Repetitive MRI of organs at risk in head and neck cancer patients undergoing radiotherapy. Clin Transl Radiat Oncol 2019;18:131-139.
2. Look D, Locker D. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 1970;41:250-251.
3. Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn Reson Med 2008;60(2):320-329.
4. Blystad I, Warntjes JB, Smedby O, Landtblom AM, Lundberg P, Larsson EM. Synthetic MRI of the brain in a clinical setting. Acta Radiol 2012;53(10):1158-1163.
5. Tanenbaum LN, Tsiouris AJ, Johnson AN, Naidich TP, DeLano MC, Melhem ER, Quarterman P, Parameswaran SX, Shankaranarayanan A, Goyen M, Field AS. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. AJNR Am J Neuroradiol 2017;38(6):1103-1110.
6. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495(7440):187-192.
7. Korzdorfer G, Kirsch R, Liu K, Pfeuffer J, Hensel B, Jiang Y, Ma D, Gratz M, Bar P, Bogner W, Springer E, Lima Cardoso P, Umutlu L, Trattnig S, Griswold M, Gulani V, Nittka M. Reproducibility and Repeatability of MR Fingerprinting Relaxometry in the Human Brain. Radiology 2019;292(2):429-437.
8. Heule R, Ganter C, Bieri O. Triple echo steady-state (TESS) relaxometry. Magn Reson Med 2014;71(1):230-237.
9. Madore B, Jerosch-Herold M, Chiou JG, Cheng CC, Guenette JP, Mihai G. A relaxometry method that emphasizes practicality and availability. Magn Reson Med 2022;88(5):2208-2216.
10. Gurney-Champion OJ, Mahmood F, van Schie M, Julian R, George B, Philippens MEP, van der Heide UA, Thorwarth D, Redalen KR. Quantitative imaging for radiotherapy purposes. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2020;146:66-75.
11. Kaza E, Guenette JP, Guthier CV, Hatch S, Marques A, Singer L, Schoenfeld JD. Image quality comparisons of coil setups in 3T MRI for brain and head and neck radiotherapy simulations. J Appl Clin Med Phys 2022:e13794.
12. Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med 2005;54(3):507-512.
Figures