2131
Comparative Evaluation of Metabolite Composition in Brain Tumor Epilepsy Patients1Center for Clinical Spectroscopy, Department of Radiology, Brigham and Women's Hospital , Harvard Medical School, Boston, MA, United States, 2Department of Neurology, Brigham and Women's Hospital , Harvard Medical School,, Boston, MA, United States
Synopsis
Keywords: Tumors, Spectroscopy, Brain Tumor-Related Epilepsy, Isocitrate Dehydrogenase Mutation
Brain Tumor Related Epilepsy (BTRE) is a multi-factorial condition with unclear pathophysiology. It has a robust prevalence in tumor patients with 20-40% experiencing onset seizures and a further 20-45% experiencing refractory seizures to treatment.1 This work examines the tumor microenvironment using magnetic resonance spectroscopy (MRS) to learn more about the epileptogenic mechanisms through a comparison of metabolite composition in BTRE patients and non-seizure tumor patients. Increasing knowledge about the pathophysiology of BTRE might aid the management of anti-tumor and BTRE treatment in the future.Introduction
Brain tumor-related epilepsy (BTRE) is a common comorbidity in both low- and high-grade gliomas. The risk of seizures varies between 60%-88% in low-grade gliomas (LGGs) and 40%-60% in high-grade gliomas (HGGs).2,3 Seizures occurring before and after tumor treatment have a strong impact on the quality of life for many patients. In BTRE, the presence of epilepsy is considered a risk factor for long-term disability.1 Moreover, BTRE is often drug-resistant, causing long-term adverse effects on social, professional, and family life. Although BTRE is exceptionally common in patients with gliomas, the mechanisms of epileptogenesis are not fully understood. This study hypothesized that analyzing quantified metabolites using MRS may provide novel insights into epileptogenesis in gliomas and the function of metabolites as potential biomarkers of seizure presentation. First, we compared the metabolic profiles of brain tumors of patients with seizures and those without. Second, we focused on post-operative metabolite variations to gain insight into the tumor microenvironment in treatment resistant BTRE patients with isocitrate dehydrogenase mutations (IDH M).Methods
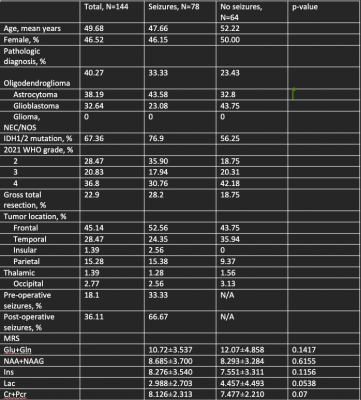
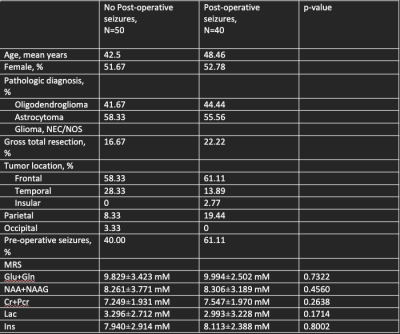
Data Collection: We retrospectively investigated patient demographics (gender, age,) and tumor characteristics (location, pathology, and genetic features) in patients with gliomas. The presence of pre-operative and post-operative seizures, tumor location, integrated molecular and histopathologic diagnosis, IDH1/2 status, and extent of resection was extracted from patient charts. MRS scans were acquired at 3T (Siemens Skyra) using point-resolved spectroscopy (TR/TE=2000/30 ms). In the first stage of the study, we divided patients solely on seizure presentation (Table 1). In the second stage of the study, we focused on IDH M patients with post-operative seizures, for which predictive biomarkers are of greatest clinical interest (Table 2). The postoperative patient cohort included patients who had both pre-and post-operative seizures, and patients who only had post-operative seizures. The patient cohort without post-operative seizures included patients with no seizure presentation and patients with only pre-operative seizures.Data processing and quantification: MRS data were reconstructed by OpenMRS lab and LCmodel to quantify the following metabolites: creatine and phosphocreatine ( Cr+Pcr), myoinositol (Ins), lactate (Lac), glutamine and glutamate (Gln+Glu), and n-acetyl aspartate (NAA+NAAG). All scans were processed using the coil correction pipeline in OpenMRSlab. In GraphPad Prism 8, all metabolite concentrations were processed through the Shapiro-Wilk test and were determined to be not normally distributed. Therefore, through exploratory statistical analysis via the Mann-Whitney Test, metabolite variations were extracted between seizure patients and non-seizure patients and between IDH M patients with and without post-operative seizures.
Results and Discussion
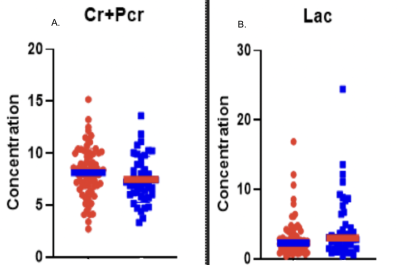
This study analyzed 700 scans and through filtering of patient data and MRS parameters (FWHM<40 Hz) 435 scans were selected. These scans corresponded to 144 patients, 78 of whom had seizures at presentation. 76% of the patients were IDH M, in concordance with the established correlation between increased seizure frequency in IDH M patients as compared to wild-type patients (WT). Most of the patients with seizure presentation had frontal and temporal tumor locations. MRS metabolite analysis detected no significant metabolites. Additionally, some metabolites indicated a trend; we observed higher Cr+Pcr, and reduced Lac, in tumor patients with seizure presentation. The previous studies4,5 found increased concentrations of total creatine, N-acetyl aspartate, glutamate, and lactate associated with epilepsy in glioblastomas. These findings were not corroborated in the current study with a greater proportion of lower-grade IDH M diffuse gliomas.IDH M patients experience greater seizure frequency pre-operatively and peri-operatively. Moreover, IDH M is the predictive biomarker of post-operative seizure outcomes.6 Thus, it was clinically relevant to observe potential metabolite changes between IDH M patients with refractory seizures compared to those without. Some studies7,8 conducted on IDH M BTRE patients report significant alterations in glutamate concentration, while others report glutamate as insignificant, and N-acetyl aspartate as significant in those with pre-operative seizures (corresponding to the control group in this study). In our analysis of IDH M patients, we found insignificant variations in metabolites.
Conclusion
This study examined glioma metabolic profiles using MRS to further investigate the role of metabolites as potential biomarkers of seizure presentation. We detected higher Cr+Pcr and reduced Lac in BTRE patients. In our IDH M cohort, we did not find metabolic differences when compared to those with post-operative seizures and those without post-operative seizures. Due to the multi-factorial nature of BTRE, the validity and prognostic value of MRS biomarkers remain to be established.Acknowledgements
The authors would like to thank the Dana-Farber Cancer Institute Center for Neuro-Oncology and the Brigham and Women’s Hospital radiology staff for their contributions to patient care.References
(1) Maschio, M. (2012). Brain tumor-related epilepsy. Current neuropharmacology, 10(2), 124-133.
(2) Ruda, R., Bello, L., Duffau, H., & Soffietti, R. (2012). Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro-oncology, 14(suppl_4), iv55-iv64
(3) Vecht, C. J., Kerkhof, M., & Duran-Pena, A. (2014). Seizure prognosis in brain tumors: new insights and evidence-based management. The oncologist, 19(7), 751-759.
(4) Nakamura, Y., Inoue, A., Nishikawa, M., Ohnishi, T., Yano, H., Kanemura, Y., ... & Kunieda, T. (2022). Quantitative measurement of peritumoral concentrations of glutamate, N-acetyl aspartate, and lactate on magnetic resonance spectroscopy predicts glioblastoma-related refractory epilepsy. Acta Neurochirurgica, 1-14.
(5) Hashiguchi, M., Tanaka, K., Nagashima, H., Fujita, Y., Tanaka, H., Kohta, M., ... & Sasayama, T. (2022). Glutamic Acid and Total Creatine as Predictive Markers for Epilepsy in Glioblastoma by Using Magnetic Resonance Spectroscopy Before Surgery. World Neurosurgery, 160, e501-e510.
(6) Jiang, H., Liu, B., Deng, G., Yuan, F., Tan, Y., Yang, K., ... & Chen, Q. (2020). Short-term outcomes and predictors of post-surgical seizures in patients with supratentorial low-grade gliomas. Journal of Clinical Neuroscience, 72, 163-168
(7) Nakae, S., Kumon, M., Murayama, K., Ohba, S., Sasaki, H., Inamasu, J., ... & Hirose, Y. (2021). Association of preoperative seizures with tumor metabolites quantified by magnetic resonance spectroscopy in gliomas. Scientific reports, 11(1), 1-8.
(8) You, G., Sha, Z., & Jiang, T. (2012). The pathogenesis of tumor-related epilepsy and its implications for clinical treatment. Seizure, 21(3), 153-159.