2128
Multiparametric MRI-based fusion radiomics for preoperatively predicting TERT promoter mutation status and survival in glioblastoma patients
Hongbo Zhang1, Hanwen Zhang2, Beibei Zhou3, Yuze Zhang1, Lei Wu1, Yi Lei2, and Biao Huang1
1Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China, 2Department of Radiology, The First Affiliated Hospital of Shenzhen University, Health Science Center, Shenzhen Second People's Hospital, Shenzhen, China, 3Department of Radiology, Department of Radiology,The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
1Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China, 2Department of Radiology, The First Affiliated Hospital of Shenzhen University, Health Science Center, Shenzhen Second People's Hospital, Shenzhen, China, 3Department of Radiology, Department of Radiology,The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
Synopsis
Keywords: Tumors, Radiomics
Radiomics uses computer software to mine massive quantitative image features from medical imaging images and then screens the most valuable radiomics features using statistical and/or machine learning methods. Furthermore, it is used to parse clinical information for disease characterisation, tumour grading and staging, efficacy evaluation, and prognosis prediction. In our study, we demonstrated that multiparametric MRI-based fusion radiomics model is an effective preoperative non-invasive method to predict telomerase reverse transcriptase promoter mutations and progression-free survival in glioblastoma patients.Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumour in adults, accounting for approximately 49.1% of all cases[1]. GBM with telomerase reverse transcriptase (TERT) promoter mutations is highly invasive, has a high recurrence rate, and requires a large range of Intraoperative radiotherapy[2].However, intraoperative frozen pathology is unlikely to reveal molecular genetic information about the tumour. Therefore, we hypothesised that the radiomic profiles from brain MRI could represent the underlying TERT promoter mutation and its prognostic importance in patients with GBM.Purpose
We propose a multiparametric MRI-based fusion radiomics model (MMFR) for TERT promoter mutations and progression-free survival (PFS) non-invasive preoperative prediction in GBM patients and validate with an external dataset.Materials and Methods
In total, 195 eligible patients with GBM were retrospectively recruited from two hospitals (108 in the training cohort and 87 in the external validation cohort). Quantitative imaging features were extracted from each patient's T1-weighted, contrast-enhanced T1-weighted, and T2-weighted images before surgery.The final feature set included 3442 features per MR sequence (T1WI, T1CE, and T2WI), resulting in a total of 10326 radiomics features per patient.By selecting features using a coarse-to-fine feature selection strategy, four radiomics signature models were constructed based on each of the three MRI sequences and their combination for TERT promoter mutation status and progression-free survival (Fig.1).we calculated the receiver operating characteristic curve (ROC) and area under curve (AUC) to evaluate the performance of the TERT promoter mutations prediction models. Harrell’s concordance index (C-index), and Kaplan–Meier curves were used to evaluate the performance of the prognostic prediction models.All statistical and machine-learning algorithms were implemented using R software.Results
TERT promoter mutations status was best predicted by MMFR, with a training cohort AUC of 0.897(95% CI:0.835-0.960). The same external validation cohort also achieved stable and optimal prediction results, with an AUC of 0.855(95% CI:0.767-0.943) (Fig.2). Similarly, MMFR showed better performance in predicting patient PFS, with a Cindex of 0.649 (95%CI:0.551-0.747,p=0.003), compared to the single-sequence radiomics signature(Fig.3,Table 1).Conclusion
In conclusion, the current preliminary study suggests that MMFR is a potential tool for predicting TERT promoter mutations and PFS in GBM patients. With further clinical research, a radiomics approach can be used to build a predictive model that combines multiparametric MRI and clinical information.Acknowledgements
No acknowledgement found.References
[1]. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol 23:iii1-iii105.
[2]. Lee Y, Koh J, Kim SI et al (2017) The frequency and prognostic effect of TERT promoter mutation in diffuse gliomas. Acta Neuropathol Commun 5:62
Figures
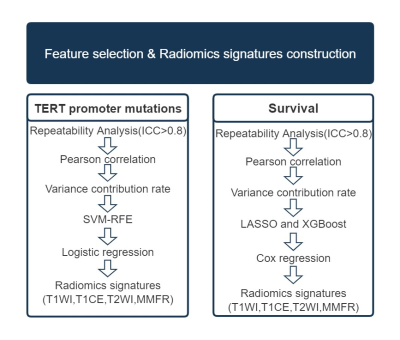
Fig.1 The workflow of radiomics feature selection and radiomics
signatures construction for TERT promoter mutation and survival prediction.
ICC, intraclass
correlation coefficient; LASSO, least absolute shrinkage and selection operator;
SVM-RFE,
support vector
machine-recursive feature elimination; TERT, telomerase reverse transcriptase;
XGBoost, extreme gradient boosting
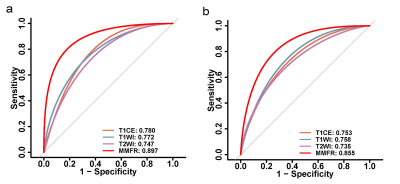
Fig.2 ROC curves among different radiomic signatures and different
models. a) ROC
curves predicted by single-sequence models and MMFR for TERT promoter mutations
in the training cohort. b) ROC curves predicted by single-sequence
models and MMFR for TERT promoter mutations in the validation cohort.
MMFR,
multiparametric MRI-based fusion radiomics model; TERT, telomerase reverse
transcriptase
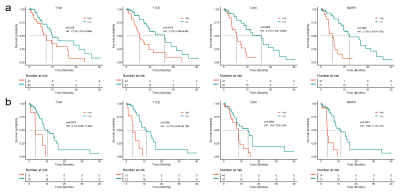
Fig.3 Kaplan-Meier analyses of PFS. a) Kaplan-Meier curves of
single-sequence model and MMFR for PFS prediction in the training cohort. b)
Kaplan-Meier curves of single-sequence modal and MMFR for PFS prediction in the
validation cohort.
PFS, progression-free
survival; MMFR, multiparametric MRI-based fusion radiomics model; TERT,
telomerase reverse transcriptase.
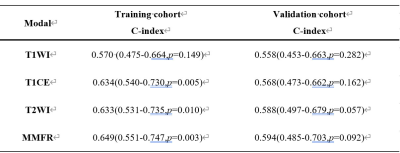
Table 1 The performance of the MMFR and
single-sequence models for PFS prediction in training cohort and external
validation cohorts
C-index,concordance
index;MMFR, multiparametric MRI-based fusion radiomics model;PFS,progression-free
survival.
DOI: https://doi.org/10.58530/2023/2128