2121
Evaluation of relaxometry in differentiating recurrence and necrosis of high-grade glioma after radiotherapy using synthetic MR
LIU YANLING1, CUI YUELONG1, NIU ZHEN1, GUO JINXIA2, ZHANG SHASHA1, ZHANG JUNLI1, and WANG YONG1
1Anyang District Hospital, Anyang City, China, 2GE Healthcare, Beijing, China
1Anyang District Hospital, Anyang City, China, 2GE Healthcare, Beijing, China
Synopsis
Keywords: Tumors, Cancer
This study is to utilize the relaxometry generated by unenhanced and Gd-enhanced synthetic MRI to identify the recurrence and necrosis in patient with high grade glioma after radiotherapy. The results indicated enhanced T1 was significantly shortened in tumor and peripheral edema region for recurrent tissue in compare with necrosis and showed well ability of differentiationIntroduction
Chemoradiotherapy is usually necessary for patients with high grade glioma (HGG), but high recurrence rate can be found even with careful surgery and treatment [1,2]. The identification of postoperative recurrence and radiation induced brain injury remains a major challenge, since the enhancement in conventional weighted MRI images for recurrent lesion behave similar to that on the radiation necrotic tissue [3]. Relaxometry are considered associated with the tissue components and potentially provides quantitative and sensitive tumor characterization. Studies with T1 or T2 in tumor grading and differentiation have been reported [4,5]. Synthetic MRI is the promising technique developed recently for simultaneously generating relaxometry map in clinical tolerant time. This study aims to explore the possibility of synthetic MRI in identifying recurrence and radiation necrosis for HGG patients after radiotherapy.Methods
36 patients (age: 51±15 years old; male/female: 19/17) confirmed HGG and went through radiotherapy were collected. . 21 patients were diagnosed as recurrent by secondary operation or MRI follow-up, and 15 patients were diagnosed as radiation necrosis. Conventional T1-weighted (T1W), T2-weighted (T2W), and synthetic MRI with magnetic resonance image compilation (MAGiC) were acquired on a 3.0T scanner (SIGNA Architect, GE Healthcare, Milwaukee, WI) for all subjects during the follow up and the parameters for MAGiC were as follows: field of view (FOV) = 240 mm×180 mm, TR/TE1/TE2 = 4000/19/95.2ms, matrix = 320×256, slice thickness/gap = 5mm/2.5mm, echo train length = 16, bandwidth = 27.78 kHz, number of slices = 20 and total imaging time = 3min 28s. Enhanced imaging of same sequences were scanned after injecting Gadopentetate dimeglumine (Gd-DTPA, Consun, Guangdong, China) with a single of 02ml/kg, at a rate of 2.0ml/s. Imaging done in 1~2 month after 3 times of radiation therapies was used for analysis. . The MAGiC data were then processed with SytheticMR (version 8.04, LinkÖping, Sweden) to obtain the parametric T1/T2 relaxometry map. Region of interests (ROIs) with size of 25-35mm² were manually delineated in unenhanced (T1) and enhanced (T1+C) relaxometry map in the area of enhancing tumor core and peripheral edema using the enhanced T1W as the reference separately by two radiologist both with 12 experience on brain tumor imaging (Figure 1 & 2). The mean of two measures in each region were used as the final quantification for comparison between radiation necrosis and recurrence group with Student-t test or Mann-Whitney U test after the normality and homogeneity test. Receiver operator characteristic (ROC) curve analysis were also done. All statistics were conducted with R language (version 4.0). P <0.05 indicated significant difference.Results
T1 was significantly shortened in the Gd enhanced imaging in tumor region and peripheral edema for recurrence group while only in tumor region for necrosis group. T2 in tumor region for recurrence group was significantly decreased. In compare with radiation necrosis lesion, Significant lower T1+C relaxometries in HGG recurrence lesions were showed both in tumor and peripheral edema (Figure 3, Table 1), but no significant differences for other relaxometry quantifications. The AUC for T1+C in HGG recurrence and radiation necrosis lesions was respectively 0.9571 (tumor, sensitivity = 1, specificity = 0.8667) and 0.8984 (edema, sen. = 1, spe. = 0.7333) (Figure 4).Discussion
As paramagnetic contrast, gadolinium (Gd) shortened the T1 and T2 relaxometry when perfusing into the tissue through vessels, inducing the enhanced signal in T1W images but reduced quantification in T1 mapping. In tumor region, due to more abundant vasculature, remarkable decreasing in T1 and T2 could be found in recurrent lesion. T1 was also shortened in necrosis tumor region, which might be because of the leakage of Gd resulted from the damaged blood-brain barrier. For recurrence group, reduced T1 in edema region might mean that there was tumor cell infiltration along with angiogenesis. Since T1 in recurrence group was shortened significantly greater than that of necrosis group, it could be used to well differentiate the tumor recurrence and necrosis after radiation therapy.Summary of main finding
Enhanced T1 relaxometry in tumor and peripheral edema region of recurrent lesion was significantly shortened in compare with necrosis for patients with high grade glioma after therapy and showed well performance of differentiation of necrosis and recurrence (AUC= 0.9571, 0.8984).Acknowledgements
No acknowledgement found.References
[1] Soffetti R, Baumert BG, Bello L, et al. Guide linea on management of low-grade gliomas: report of an EFNS-EANO Task Force [J]. EurJ Neuro, 2010, 17(9):1124-1133. [2] Alexiou GA, Tsiouris S, Kyritsis AP, et al. Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities[J]. J Neurooncol, 2009, 95:1. [3] Arvinda HR, Kesavadas C, Sarma PS, et al. Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging [J]. Neuro Oncol, 2009, 95(1):87-96. [4] Goncalves FG, Serai SD, Zuccoli G. Synthetic Brain MRI: Review of Current Concepts and Future Directions[J]. Top Magn Reson Imaging, 2018, 27:387-393. [5]Ulrike Noth, Julia Tichy,Stephanie Tritt, ,et al. Quantitative T1mapping indicates tumor infiltration beyond the enhancing part of glioblastomas: NMR in Biomedicine[J]., 2019, 11(27):1-11.Figures
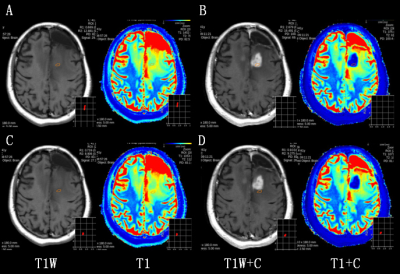
Figure 1. A 41
years old female patient with recurrent glioma (WHO grade IV) in left frontal
lobe. ROI of 25-35mm² was respectively placed on the tumor core (top) and peripheral
edema (bottom) region in no-enhanced (A, C) and enhanced (B, D) synthetic
images. ROI: region of interest.
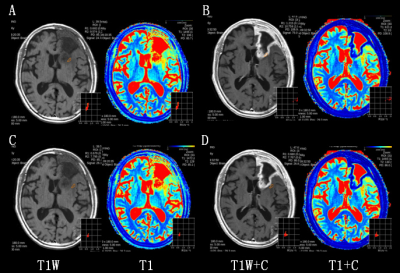
Figure 2. A 66 years old female
confirmed postoperative radiation necrosis after radiation therapy for left
frontal lobe glioma (WHO grade III). ROI of 25-35mm² was respectively placed on the tumor core (top) and
peripheral edema (bottom) region in no-enhanced (A, C) and enhanced (B, D)
synthetic images. ROI: region of interest
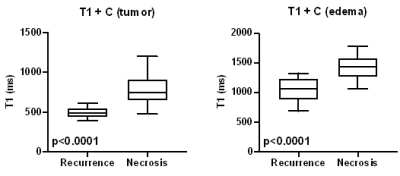
Figure 3. Boxplot for T1 relaxometry in
contrast-enhanced synthetic MR imaging in HGG recurrence and radiation necrosis
group. T1 quantification in both enhancing tumor core (A) and edema region (B)
showed significant differences.
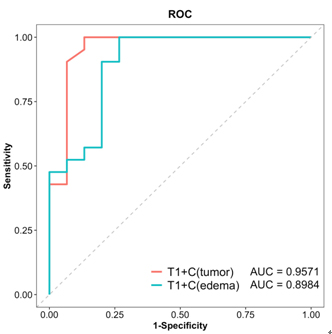
Figure 4. ROC curves of T 1 value after enhancement in tumor core and edema area
for differentiating the HGG recurrence and the
radiation necrosis. HGG: high grade glioma.
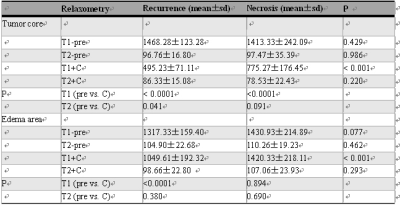
Table 1. Comparison of T1 and T2 relaxometry between HGG recurrence and radiation
necrosis group
DOI: https://doi.org/10.58530/2023/2121