2109
Neural correlates of bi-cephalic transcranial direct current stimulation in upper limb hand function post stroke1Department of Neurology, All India Institute of Medical Sciences, New Delhi, India, 2Department of NMR, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Keywords: Stroke, fMRI (task based)
Non invasive brain stimulation holds great promise in post stroke recovery in upper limb hand function and inducing neural plasticity. Transcranial direct current stimulation (tDCS) enables the alteration of cortical excitability by passing direct currents causing hypo or hyperpolarization of neuronal resting membrane potentials. Bi-cephalic tDCS with anode applied on the affected cortex especially M1 and cathode over the non-affected cortex has been used to normalize excitatory and inhibitory corticospinal networks.Background
Non invasive brain stimulation alters cortical excitability and enhances the effects of proven conventional rehabilitation treatments to improve motor function after stroke. The objective was to study the effects of transcranial direct current stimulation (tDCs) in improving hand function post stroke using clinical assessments and functional MRI.Methods
This was a randomized controlled, single blinded, outcome assessor study design. A total of 25 patients with chronic stroke (3 months to 3 years with age in the range of 18 to 75 years of age; hand muscle power MRC: 1-3; brunnstrom stage (2-4) were recruited. Patients were randomised to real (current 2mA for 20min) and sham (0 mA for 20min) groups. A tDCS system (Mind Acquity, USA) was used with the anode electrode placed over C3/C4 of lesioned cortex and stimulation given for a duration of 20 min, followed by physical therapy session for 35-40 min for 5 days a week for 4 weeks. The clinical and fMRI assessments were done at baseline, 1 and 3 months. Multi-slice (MB:2) axial blood oxygen-level dependent (BOLD) images were acquired using gradient echo planar imaging (GE-EPI) with Fat suppression (SPIR); FOV 230; 35 slices and slice thickness of 4 mm without any slice gap and TR of 1s.Data processing was carried out using standard pipeline of preprocessing (realignment, co-registration, normalization, and smoothing) of the functional images using statistical parametric mapping (ver. SPM12) and processing based on general Linear Model (GLM). The neuronal activity in response to an experimental task was obtained by specifying linear contrasts (T contrast).
Results
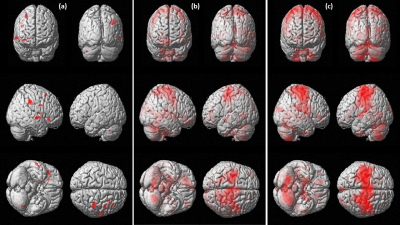
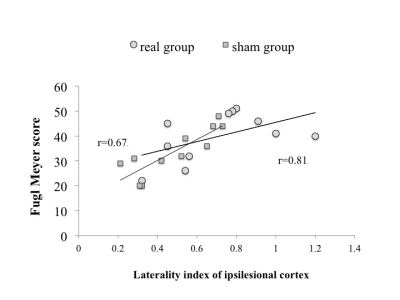
The mean age of all patients (16M, 9F) was 58.5+6.7 years and mean time of onset 2.1 years. The number of patients in the real group was n=13 and n=12 in the sham group. Fugl Meyer score (FM) and modified Barthel Index (mBI) exhibited a significant improvement on one way ANOVA at 3 months (95% CI; 5.6 to 1.2; p=0.04 in Real group; 95% CI=3.2 to 4.2; p=0.05 in Sham group). BOLD analysis revealed activation in right Brodmann area 6 (239 clusters), sensory cortex BA 2,3 (156) and inferior parietal lobule BA 40 (89) in real group (Figure 1). Laterality Index (LI) correlation with FM score revealed a trend of good recovery at 1 month in real group with Pearson’s correlation coefficient r=0.81) in comparison with the sham group (r=0.67) (Figure 2).Discussion
The use of tDCS is safe and feasible in stroke patients, without any adverse events reported. Trend of improvement was observed in FM and modified Barthel index at 4 weeks. BOLD analysis showed motor and coordination areas being active at 1 month in real group, and were more dorsal than ventral. This may be attributed to cortical reorganization resulting in better functional recovery. The potential of online mode of tDCS (brain stimulation coupled with rehabilitation or neuroimaging) with lower costs, facilitates its easier clinical integration.Conclusion
A stronger activation of ipsilesional premotor and primary motor regions (BA4,6) in the real group suggest functional recovery by the use of tDCS in post stroke patients.Acknowledgements
Funding from ICMR, CARE-DAT is acknowledgedReferences
1. Feigin VL, Farounzafar MH, Krishnamurthy R, Mensah GA, Connor M et al. Global and regional burden of stroke during 1990-2010: findings from Global Burden of Diseases 2010. Lancet 2014;383:245-254.
2. Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 2010;75:2176-2184.
3. Calautti C, Baron JC. A review: Functional Neuroimaging Studies of Motor Recovery After Stroke in Adults. Stroke 2003;34(6):1553-1556.
4. Auriat AM, Neva JL, Peters S, Feriis JK, Boyd LA. A review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Frontiers in Neurology 2015;6:226-230.