2103
Ultra-high field MRI sequences for clot characterization in acute ischemic stroke1Division of Diagnostic and Interventional Neuroradiology, HUG Geneva University Hospitals, Geneva, Switzerland, 2Radiology and Medical Informatics, University of Geneva, Geneva, Switzerland, 3Ecole Polytechnique Fédérale de Lausanne (EPFL), Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 4Department of Physiology, University of Galway, Galway, Ireland, 5CURAM, Sciene Foundation Ireland (SFI) Centre for Research in Medical Devices, University of Galway, Galway, Ireland, 6Division of Neuroradiology, Klinik Hirslanden, Zurich, Switzerland, 7Swiss Neuroradiology Institute, Zurich, Switzerland, 8Division of Neuroradiology, University of Essen, Essen, Germany
Synopsis
Keywords: Stroke, Stroke
In acute ischemic stroke, the composition of the clot occluding the arteries is associated with the underlying pathophysiology and with the response to treatment. Innovative MRI sequences aim to improve the ability to recognize clot composition from neuroimaging signs. Using ultra-high field MR imaging of clot analogs, we demonstrate the ability of R2* map to distinguish between red blood cells-rich and fibrin-rich compositions. This technique can also be used for the volumetric analysis of formalin fixed clots. Further studies with intermediate clot compositions and improved phantoms will reveal the full potential of MRI sequences for depicting clot composition.INTRODUCTION
In acute ischemic stroke (AIS), the composition of the clot occluding the arteries is associated with the underlying pathophysiology and with the response to treatment. Information from clinical imaging about clot composition could support decisions regarding secondary preventive measures and help design new treatment strategies and devices. So far, in clinical MR scans of patients with AIS, it was established that the susceptibility vessel sign (SVS), when present in T2* gradient echo sequences (GRE) or susceptibility weighted imaging (SWI), is associated with red blood cells (RBCs)-rich clots vulnerable to treatment 1. Clots that do not display SVS are rich in fibrin/platelets and more often require the use of combined therapy 2. Susceptibility measurements performed with quantitative susceptibility mapping (QSM) 3 and machine learning algorithms based on GRE images 4 can help predict stroke etiology. More exploration is necessary for identifying sequences that can depict with accuracy clot composition. In addition, quantitative volumetric techniques, able to reach beyond conventional histopathology, could help improve the current understanding about clot organization. Ultra-high field MRI is a valuable evaluation technique for clinical purposes, due to the enhanced sensitivity. It can also provide useful information for the validation of histopathological findings, when used for the ex vivo imaging of biological tissues 5 6.In this study we illustrate, using clot analogs in vitro, the ability of several MRI sequences at 7T to distinguish between fibrin-rich and RBCs-rich compositions.
METHODS
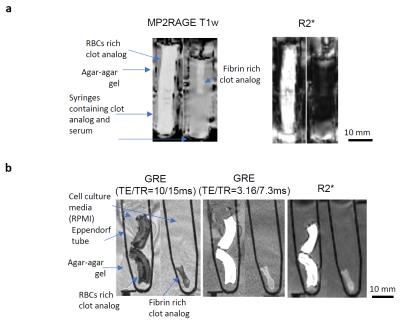
For this purpose, clot analogs with RBCs-rich and fibrin-rich compositions were prepared as previously described 7. The clots were imaged in triplicates, in fresh state and after fixation in formalin. The clots underwent 1H magnetic resonance imaging scans with a 7 Tesla/68 cm MR scanner (Siemens Medical Solutions, Erlangen, Germany), using 32-channel receive coil (NOVA Medical Inc., MA) with a single channel volume transmit coil. 3D T1-weighted MR images were acquired using MP2RAGE 8 (TE/TR = 3.37/5000 ms, TI1/TI2 = 800/2700 ms, α1/α2 = 4°/5°,0.6 mm isotropic resolution, bandwidth = 240 Hz/Px). 3D high resolution gradient-echo (GRE) images were acquired with two parameter settings (TE/TR= 10/15ms or 3.16/7.3ms, 0.3 mm isotropic resolution, FA = 4 °, bandwidth = 240 Hz/Px) to enhance the contrast for blood clot samples. Quantitative R2* maps were acquired with 3D multi-echo GRE sequence with monopolar readout (8 TEs ranging from 4.2 to 35.12ms, 0.75mm isotropic resolution, GRAPPA factor of 4, in-plane resolution = 0.7 mm, slice thickness = 0.8 mm, receiver bandwidth = 340 Hz/pixel, TR = 38 ms with no flow compensation, FA = 15 °) with the reconstruction method of Computationally Efficient Combination of Multi-channel Phase Data From Multi-echo Acquisitions (ASPIRE) 9. Clot segmentation was performed with 3D Slicer 10. Histograms representing the probability of signal intensity distribution were derived from clot segmentations with MATLAB.RESULTS
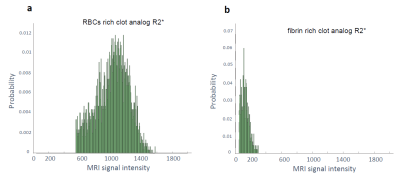
The signal intensity distribution for clot analogs imaged with R2* map is exemplified in Figure 1. The distribution was reproducible for compositionally identical clot analog samples. Compositionally different clots, in fresh (as prepared) state, displayed distinct ranges of signal intensity. The RBCs rich clot analogs appear hyperintense in R2*. The fibrin rich clots appear hypointense in R2*. Relevant MR images are presented in Figure 2a. MR can image as well formalin-fixed clot analogs – Figure 2b. For formalin-fixed clot analogs, GRE sequences are useful for visualizing the compact RBCs core and for delineating the clot, while R2* map can differentiate between the RBCs-rich and fibrin-rich compositions.DISCUSSION
Among the investigated sequences at 7 T, the R2* map helps the most to differentiate between RBCs-rich and fibrin-rich compositions in clot analogs in vitro, in fresh state and in formalin-fixed state. GRE sequences have the potential of distinguishing the compact RBCs regions in RBCs rich clots after fixation. In perspective, optimization of MP2RAGE sequences could also allow clot depiction according to composition.CONCLUSION
Ultra-high field MRI can be used to characterize clots in vitro. Further studies, using series of clots with incremental changes in composition (various RBCs-to-fibrin ratios) and phantoms that are mimicking closely the in vivo conditions, will reveal in detail the potential of MRI for clot composition characterization.Acknowledgements
The project has been funded by a grant of the Swiss National Science Foundation (32003B_182382), by a grant of University Hospitals of Geneva, Radiology Department Startup fund, and a grant of the Science Foundation Ireland (13/RC/2073_2).References
1. Benson JC, Kallmes DF, Larson AS, Brinjikji W. Radiology-Pathology Correlations of Intracranial Clots: Current Theories, Clinical Applications, and Future Directions. Am J Neuroradiol. 2021;42(9):1558-1565. doi:10.3174/ajnr.A7249
2. Darcourt J, Garcia C, Phuong DM, Michelozzi C, Bellanger G, Adam G, Roques M, Januel AC, Tall P, Albucher JF, Olivot JM, Bonneville F, Payrastre B, Cognard C. Absence of susceptibility vessel sign is associated with aspiration-resistant fibrin/platelet-rich thrombi. Int J Stroke Off J Int Stroke Soc. 2021;16(8):972-980. doi:10.1177/1747493020986626
3. Chen J, Zhang Z, Nie X, Xu Y, Liu C, Zhao X, Wang Y. Predictive value of thrombus susceptibility for cardioembolic stroke by quantitative susceptibility mapping. Quant Imaging Med Surg. 2022;12(1):550-557. doi:10.21037/qims-21-235
4. Chung J, Kim Y, Cha J, Choi E, Kim BM, Seo W, Kim G, Bang OY. Characterization of clot composition in acute cerebral infarct using machine learning techniques. Ann Clin Transl Neurol. 2019;6(4):739-747. doi:10.1002/acn3.751
5. Harteveld AA, Denswil NP, Siero JCW, Zwanenburg JJM, Vink A, Pouran B, Spliet WGM, Klomp DWJ, Luijten PR, Daemen MJ, Hendrikse J, van der Kolk AG. Quantitative Intracranial Atherosclerotic Plaque Characterization at 7T MRI: An Ex Vivo Study with Histologic Validation. Am J Neuroradiol. 2016;37(5):802-810. doi:10.3174/ajnr.A4628
6. Alkemade A, Pine K, Kirilina E, Keuken MC, Mulder MJ, Balesar R, Groot JM, Bleys RLAW, Trampel R, Weiskopf N, Herrler A, Möller HE, Bazin PL, Forstmann BU. 7 Tesla MRI Followed by Histological 3D Reconstructions in Whole-Brain Specimens. Front Neuroanat. 2020;14:536838. doi:10.3389/fnana.2020.536838
7. Fitzgerald ST, Liu Y, Dai D, Mereuta OM, Abbasi M, Larco JLA, Douglas AS, Kallmes DF, Savastano L, Doyle KM, Brinjikji W. Novel Human Acute Ischemic Stroke Blood Clot Analogs for In Vitro Thrombectomy Testing. Am J Neuroradiol. 2021;42(7):1250-1257. doi:10.3174/ajnr.A7102
8. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage. 2010;49(2):1271-1281. doi:10.1016/j.neuroimage.2009.10.002
9. Eckstein K, Dymerska B, Bachrata B, Bogner W, Poljanc K, Trattnig S, Robinson SD. Computationally Efficient Combination of Multi-channel Phase Data From Multi-echo Acquisitions (ASPIRE): Combination of Multi-Channel Phase Data from Multi-Echo Acquisitions (ASPIRE). Magn Reson Med. 2018;79(6):2996-3006. doi:10.1002/mrm.26963
10. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323-1341. doi:10.1016/j.mri.2012.05.001
Figures