2102
Assessing cerebral oxygen metabolism changes in preeclampsia using voxel-based morphometry of oxygen extraction fraction (OEF) maps in MRI1Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China, 2Department of Radiology, Weill Cornell Medical College, New York, NY, United States, 3Department of Radiology, Jinan Maternal and Child Care Hospital, Jinan, China, 4Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Synopsis
Keywords: Stroke, Hypertension
The objective of this study was to analyze the different brain oxygen metabolism statuses in preeclampsia. Furthermore, we also investigated the influencing factors that affect cerebral oxygen metabolism in preeclampsia. Forty-nine preeclampsia patients, forty nonpregnant healthy controls (NPHCs) and twenty-two pregnant healthy controls (PHCs) were included in this study. Brain OEF values were computed using quantitative susceptibility mapping (QSM) plus quantitative blood oxygen level‐dependent magnitude-based OEF mapping (QSM+qBOLD, or QQ). Voxel-based morphometry (VBM) was applied to investigate the differences of OEF values in brain regions among groups.Abstract
IntroductionHypertensive disorders of pregnancy (HDP) remain one of the main causes of pregnancy-related maternal and fetal morbidity and mortality worldwide 1. HDP includes gestational hypertension, preeclampsia and eclampsia 2. Hypoxia is the key phenomenon in preeclampsia and can lead to abnormal cerebral oxygen metabolism 3. Oxygen extraction fraction (OEF) and the cerebral metabolic rate of oxygen (CMRO2) are key brain physiological parameters for identifying high-risk cerebrovascular patients and understanding cerebral function 4. Threfore, for preeclampsia, an accurate, safe and sensitive neuroimaging method is needed to directly evaluate cerebral oxygen metabolism. In this study, we used QQ‐based OEF mapping to evaluate brain metabolic oxygen consumption. In addition, voxel-based morphometry (VBM) can detect brain injury at the voxel level 5,6 and detect brain abnormalities more sensitively. Since preeclampsia is related to hypertension, obesity, age and other factors, we also analyzed the relationship between OEF values and clinical characteristics.
Materials
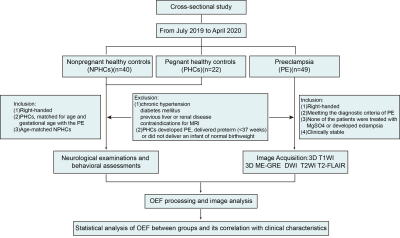
This study was approved by the institutional review board of Jinan Maternity and Child Care Hospital Affiliated with Shandong First Medical University. Forty-nine PE patients, forty NPHCs and twenty-two PHCs were enrolled in this cross-sectional study. All participants were informed of the detailed experimental procedures and signed informed consent forms. All study participants were scanned on a 1.5-T MR scanner. The brain scanning protocol consisted of a 3D T1-weighted high resolution sequence for anatomic structureand a 3D multiecho gradient echo (ME-GRE) sequence for QSM. In addition, T2-weighted turbo spin echo (TSE), T2-weighted fluid attenuated inversion recovery and diffusion-weighted were acquired to detect brain abnormalities. Brain OEF values were computed using quantitative susceptibility mapping (QSM) plus quantitative blood oxygen level‐dependent magnitude-based OEF mapping (QSM+qBOLD, or QQ). Voxel-based morphometry (VBM) was applied to investigate the differences of OEF values in brain regions among groups. Pearson correlation analysis was used to test the correlations between OEF values and some clinical indicator values in PE patients. False Discovery Rate (FDR) correction was used for multiple comparison correction.
Results
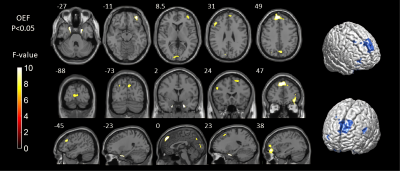
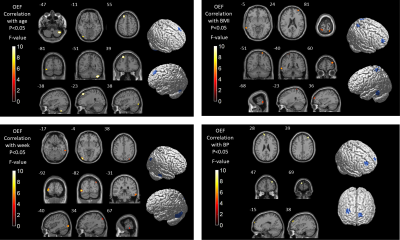
Detailed study participants information is summarized (Figure 1). Among the three groups, the OEF values were significantly different in extensive brain areas, including the parahippocampus, multiple gyri of the frontal lobe, calcarine, cuneus and precuneus (P<0.05)(Figure2). Correlation analysis revealed that in preeclampsia, the OEF values of some brain regions (mainly involving the frontal, occipital and temporal gyrus) positively correlated with age, gestational week, body mass index (BMI) or mean blood pressure (BP)(Figure3).
Discussion
Using whole-brain voxel-based morphometry analysis, we compared the OEF values among three groups. We found that the OEF values of some brain regions of preeclampsia patients were higher than those of PHCs and NPHCs main in the frontal lobe, parahippocampal gyrus, calcarine, cuneus and precuneus. We inferred that the increased OEF value was the complementary response for cerebral ischemia and hypoxia in preeclampsia. At the same time, we analyzed the relationship between OEF values and clinical characteristics in preeclampsia patients. Previous studies on acute stroke have shown that OEF values are significantly increased in the ischemic area and negatively correlated with decreased cerebral blood flow 7. Therefore, we inferred that the increased OEF values of preeclampsia patients might share the same mechanism with acute stroke patients. Because conventional MRI showed no abnormalities in preeclampsia, our findings suggested that cerebral ischemia in preeclampsia might be detected earlier by observing changes in OEF values. If the continuously rising BP exceeds the limit of cerebrovascular autoregulation, which will lead to the destruction of the BBB and vasogenic brain edema and may also lead to the occurrence of PRES and RCVS 8,9. In our study, the injured brain areas were also extensive in preeclampsia, especially in the frontal, occipital and parahippocampal gyrus, which also overlapped with the PRES and RCVS. We inferred that with the progression of preeclampsia, the damage of these brain regions continues to aggravate, which may progress to PRES and RCVS. The underlying mechanisms contributing to the pathophysiology of preeclampsia are poorly understood 10. In our study, OEF values were not only positively related to mean BP but also positively related to age, BMI and gestational week, and the difference in OEF with different age, mean BP, BMI and gestational week was mainly concentrated in the frontotemporal regions. Frontotemporal connectivity and executive functions contribute to episodic memory performance 11, while episodic memory loss is the primary clinical symptom of Alzheimer's disease (AD) 12. A recent longitudinal AD study reported that early structural changes occurred in the bilateral frontoparietal, hippocampal and association occipital regions, while changes in the temporal lobe were observed in later stages 13, and the damaged brain regions of AD patients also overlap with the preeclampsia patients in this study. We inferred that preeclampsia patients may be at risk of developing AD as the disease progresses.
Conclusion
Compared with PHCs and NPHCs, the OEF values of preeclampsia patients were higher in many brain regions. These changes may be related to cerebral ischemia and hypoxia and are the result of compensatory oxygen uptake to maintain cerebral oxygen metabolism and brain function. QQ‐based OEF mapping can be used as a biological marker for brain damage detection in preeclampsia patients.
Acknowledgements
We thank all of the volunteers and patients for their participation in our study.References
1 Garovic, V. D. et al. Hypertension in Pregnancy: Diagnosis, Blood Pressure Goals, and Pharmacotherapy: A Scientific Statement From the American Heart Association. Hypertension 79, e21-e41, doi:10.1161/hyp.0000000000000208 (2022).
2 Sinkey, R. G. et al. Prevention, Diagnosis, and Management of Hypertensive Disorders of Pregnancy: a Comparison of International Guidelines. Current hypertension reports 22, 66, doi:10.1007/s11906-020-01082-w (2020).
3 Tong, W. et al. Chronic Hypoxia in Ovine Pregnancy Recapitulates Physiological and Molecular Markers of Preeclampsia in the Mother, Placenta, and Offspring. Hypertension 79, 1525-1535, doi:10.1161/hypertensionaha.122.19175 (2022).
4 Fan, A. P. et al. Quantification of brain oxygen extraction and metabolism with [(15)O]-gas PET: A technical review in the era of PET/MRI. Neuroimage 220, 117136, doi:10.1016/j.neuroimage.2020.117136 (2020).
5 Goto, M. et al. Advantages of Using Both Voxel- and Surface-based Morphometry in Cortical Morphology Analysis: A Review of Various Applications. Magn Reson Med Sci 21, 41-57, doi:10.2463/mrms.rev.2021-0096 (2022).
6 Khan, A. R., Wang, L. & Beg, M. F. Unified voxel- and tensor-based morphometry (UVTBM) using registration confidence. Neurobiol Aging 36 Suppl 1, S60-68, doi:10.1016/j.neurobiolaging.2014.04.036 (2015).
7 Fan, A. P. et al. Elevated brain oxygen extraction fraction measured by MRI susceptibility relates to perfusion status in acute ischemic stroke. J Cereb Blood Flow Metab 40, 539-551, doi:10.1177/0271678x19827944 (2020).
8 Miller, T. R., Shivashankar, R., Mossa-Basha, M. & Gandhi, D. Reversible Cerebral Vasoconstriction Syndrome, Part 1: Epidemiology, Pathogenesis, and Clinical Course. AJNR Am J Neuroradiol 36, 1392-1399, doi:10.3174/ajnr.A4214 (2015).
9 Fugate, J. E. & Rabinstein, A. A. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 14, 914-925, doi:10.1016/s1474-4422(15)00111-8 (2015).
10 Ives, C. W., Sinkey, R., Rajapreyar, I., Tita, A. T. N. & Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. Journal of the American College of Cardiology 76, 1690-1702, doi:10.1016/j.jacc.2020.08.014 (2020).
11 Blankenship, T. L., O'Neill, M., Deater-Deckard, K., Diana, R. A. & Bell, M. A. Frontotemporal function]al connectivity and executive functions contribute to episodic memory performance. Int J Psychophysiol 107, 72-82, doi:10.1016/j.ijpsycho.2016.06.014 (2016).
12 Wang, P., Zhang, H., Han, L. & Zhou, Y. Cortical function in Alzheimer's disease and frontotemporal dementia. Transl Neurosci 7, 116-125, doi:10.1515/tnsci-2016-0018 (2016).
13 Landin-Romero, R. et al. Disease-specific patterns of cortical and subcortical degeneration in a longitudinal study of Alzheimer's disease and behavioural-variant frontotemporal dementia. Neuroimage 151, 72-80, doi:10.1016/j.neuroimage.2016.03.032 (2017).
Figures