2091
Ultra High Spatial Resolution MRI of Intact Ex Vivo Multiple Sclerosis Brain1Imaging Institute, The Cleveland Clinic, Cleveland, OH, United States, 2Mellen Center, The Cleveland Clinic, Cleveland, OH, United States, 3Neurosciences, The Cleveland Clinic, Cleveland, OH, United States, 4Biomedical Engineering, The Cleveland Clinic, Cleveland, OH, United States
Synopsis
Keywords: Multiple Sclerosis, Multiple Sclerosis
Treatment options for progressive multiple sclerosis (MS) are limited and the lack of sensitive and specific MRI biomarkers is a major hurdle in therapeutic development. A number of pathophysiological processes are repeatedly found on histology but are difficult to identify accurately on MRI. Ultra-high spatial resolution MRI of intact ex vivo brain promises to help bridge the gap between MRI and pathology. We demonstrate progress towards visualizing potential biomarkers for progressive MS.INTRODUCTION
Treatment options for progressive multiple sclerosis (MS) are limited and the lack of sensitive and specific MRI biomarkers is a major hurdle in therapeutic development. A number of pathophysiological processes are repeatedly found on histology but are difficult to identify accurately on MRI. Ultra-high spatial resolution MRI of intact ex vivo brain promises to help bridge the gap between MRI and pathology. We demonstrate progress towards visualizing and quantitatively grading subpial cortical lesions and paramagnetic rim lesions (PRL), which are believed to predict disease progression. We also demonstrate the advantages of imaging intact ex-vivo tissue. Imaging-pathology correlation work typically involves tissue that has been sliced prior to imaging1,2, making it difficult to use standard radiologic tools such as 3-plane views and stack-viewing to enhance sensitivity and specificity to pathology3.METHODS
Brain tissue was procured under the Cleveland Clinic Rapid MS autopsy program with IRB-approval4. The subject was a 52 year old woman with secondary progressive MS with a disease duration of 26 years and disability requiring bilateral assistance to ambulate. The left hemisphere was fixed and stored as described by Dutta et al.4 Tissue was then prepared for imaging as described by Kim et al.5 Imaging was performed on a 7T Siemens Terra with a standard knee coil (Siemens Healthineers, Erlangen). FLASH images (170 micron isotropic voxels, TE/TR=20.5/51 msec, FA=20°) were acquired 9 times and subsequently coregistered and averaged using ANTs6. A multi-echo gradient echo (MGE, 200 micron isotropic voxels, TE=5-40 msec in steps of 5 msec, TR=47 msec, FA=35°) using the ASPIRE pulse sequence7 was used to generate magnitude, phase and quantitative T2* maps. Phase unwrapping was implemented with the ROMEO algorithm8, and susceptibility weighted images (SWI) were generated with the CLEAR-SWI package algorithm9.RESULTS
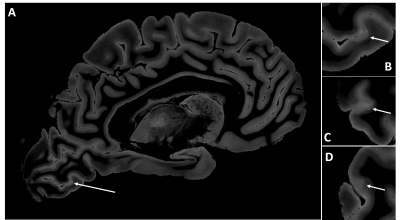
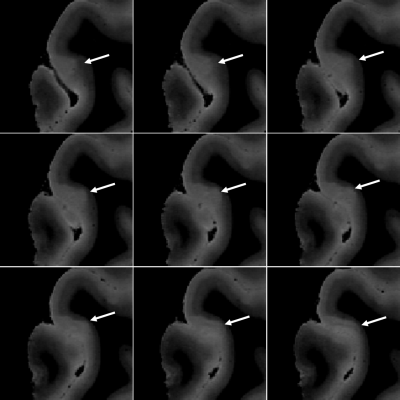
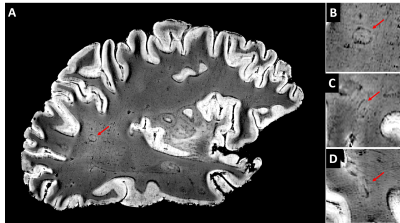
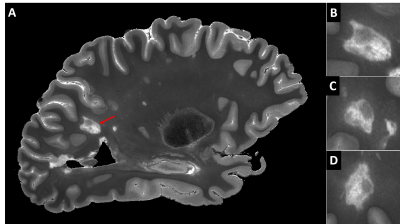
Figure 1 shows a FLASH image of a subpial lesion. A three-plane view facilitates identification of the lesion, as the borders are irregular on all three planes. Note that the contrast is inverted from that expected in vivo because of changes in relaxation rates due to fixation. Figure 2 depicts the subpial lesion from figure 1 on multiple adjacent slices. Viewing on multiple slices also enables accurate classification of the lesion as a subpial, type III lesion that does not extend to the border between gray and white matter10. Figure 3 shows a SWI image of a PRL. PRL are of particular interest as potential predictors of disease progression11. The three-plane view is crucial in this case, as the lesion is conspicuous on a sagittal view but more subtle on axial and coronal views. Figure 4 is a quantitative T2* map. Ultra high spatial resolution enables identification of heterogeneity across and within lesions.DISCUSSION
We have developed an imaging protocol that can identify a number of pathophysiologic features that are relevant to progressive MS. Important pathophysiology can be difficult to detect on in vivo MRI. For example, sensitivity to cortical lesions can be low, even with guidance from ex vivo MRI1. This work is part of a larger program to develop imaging that can accurately identify demyelination in white matter and cortex and evidence of chronic inflammation at the rims of white matter lesions in vivo. However, most ex vivo MRI studies have been limited to imaging from individual slices or even smaller pieces of tissue. The limited extent of such tissue makes it difficult to use the full range of tools used by radiologists to identify pathology, including stack viewing, dynamic adjustment of contrast and 3-plane viewing.CONCLUSION
This work is relevant to clinical practice because it promises to provide a platform for developing accurate imaging of pathophysiological processes that are relevant to progressive MS. Coming work will focus on validating the MRI findings against histopathology.Acknowledgements
Funding was provided by the 2022 Foundation of the ASNR Grant Program, NIH K23NS109328 and NIH R35NS097303. We thank Simon Robinson for developing and sharing the ASPIRE C2P pulse sequence, the ROMEO and CLEAR-SWI software packages, the ex vivo protocol and for helping adapt that protocol for use in this study.References
1. Bouman, P. M., Steenwijk, M. D., Pouwels, P. J. W., Schoonheim, M. M., Barkhof, F., Jonkman, L. E. & Geurts, J. J. G. Histopathology-validated recommendations for cortical lesion imaging in multiple sclerosis. Brain 2020; 143:2988-2997.
2. Schmierer, K., Parkes, H. G. & So, P. W. Direct visualization of remyelination in multiple sclerosis using T2-weighted high-field MRI. Neurology 2009; 72:472.
3. Waite, S., Grigorian, A., Alexander, R. G., Macknik, S. L., Carrasco, M., Heeger, D. J. & Martinez-Conde, S. Analysis of Perceptual Expertise in Radiology - Current Knowledge and a New Perspective. Front Hum Neurosci 2019; 13:213.
4. Dutta, R., Mahajan, K. R., Nakamura, K., Ontaneda, D., Chen, J., Volsko, C., Dudman, J., Christie, E., Dunham, J., Fox, R. J. & Trapp, B. D. Comprehensive Autopsy Program for Individuals with Multiple Sclerosis. J Vis Exp 2019;
5. Kim, S., Sakaie, K., Blumcke, I., Jones, S. & Lowe, M. J. Whole-brain, ultra-high spatial resolution ex vivo MRI with off-the-shelf components. Magn Reson Imaging 2021; 76:39-48.
6. Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A. & Gee, J. C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011; 54:2033-2044.
7. Eckstein, K., Dymerska, B., Bachrata, B., Bogner, W., Poljanc, K., Trattnig, S. & Robinson, S. D. Computationally Efficient Combination of Multi-channel Phase Data From Multi-echo Acquisitions (ASPIRE). Magn Reson Med 2018; 79:2996-3006.
8. Dymerska, B., Eckstein, K., Bachrata, B., Siow, B., Trattnig, S., Shmueli, K. & Robinson, S. D. Phase unwrapping with a rapid opensource minimum spanning tree algorithm (ROMEO). Magn Reson Med 2021; 85:2294-2308.
9. Eckstein, K., Bachrata, B., Hangel, G., Widhalm, G., Enzinger, C., Barth, M., Trattnig, S. & Robinson, S. D. Improved susceptibility weighted imaging at ultra-high field using bipolar multi-echo acquisition and optimized image processing: CLEAR-SWI. Neuroimage 2021; 237:118175.
10. Bo, L., Vedeler, C. A., Nyland, H. I., Trapp, B. D. & Mork, S. J. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 2003; 62:723-732.
11. Absinta, M., Sati, P., Schindler, M., Leibovitch, E. C., Ohayon, J., Wu, T., Meani, A., Filippi, M., Jacobson, S., Cortese, I. C. & Reich, D. S. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126:2597-2609.
Figures