2077
Symptomatic cerebrospinal fluid HIV escape: a quantitative MR-based assessment
Serena Capelli1, Anna Caroli1, Giulio Pezzetti2, Francesca Ferretti3, Paola Cinque4, and Simonetta Gerevini2
1Bioengineering Department, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Ranica (BG), Italy, 2Department of Neuroradiology, ASST Papa Giovanni XXIII, Bergamo, Italy, 3Lewisham and Greenwich NHS trust, London, United Kingdom, 4Unit of Infectious Diseases, San Raffaele Scientific Institute, Milano, Italy
1Bioengineering Department, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Ranica (BG), Italy, 2Department of Neuroradiology, ASST Papa Giovanni XXIII, Bergamo, Italy, 3Lewisham and Greenwich NHS trust, London, United Kingdom, 4Unit of Infectious Diseases, San Raffaele Scientific Institute, Milano, Italy
Synopsis
Keywords: Infectious disease, Infectious disease, HIV
This study aimed at quantitatively assessing T1-weighted, FLAIR and DWI brain alterations in 14 patients with cerebrospinal fluid HIV escape (HIV-ESC) and 7 patients with untreated HIV encephalitis (HIV-ENC), versus 11 HIV patients without neurological problems and 12 HIV-negative controls. HIV-ESC and HIV-ENC patients showed significantly higher ADC in WM and GM and increased WM FLAIR signal than the other groups. In HIV-ESC patients, the heterogeneous GM volume was negatively correlated with MRI time from infection. WM FLAIR hyperintensity in HIV-ESC and HIV-ENC patients may reflect vasogenic edema, with mass effect depending on its degree and possible underlying brain atrophy.Introduction
Despite contemporary systemic combined antiretroviral therapy (cART) being highly successful in controlling HIV replication in the central nervous system (CNS), some cases of HIV persistence in CNS have been increasingly observed. Symptomatic cerebrospinal fluid (CSF) HIV escape is defined as the detection of HIV-RNA in CSF overcoming the replication in plasma in patients complaining of new onset neurological problems, despite cART.1 Clinical and MRI presentation resemble HIV encephalitis in untreated AIDS presenters, with progressing neurological impairment and cognitive deterioration. Typical MRI findings are white matter abnormalities, mainly consisting of hyperintense signal alterations in T2 and FLAIR sequences.2 The aim of this study was to quantitatively assess MR brain alterations in HIV CSF Escape (HIV-ESC) and cART-untreated HIV encephalitis (HIV-ENC), as compared with treated HIV-positive patients with no neurological problems (HIV) and HIV-negative controls (CTRL).Methods
Brain MRI scans from 14 patients with HIV-ESC (49[43-54] years, 64% males), 7 patients with HIV-ENC (48[46-50] years, 100% males), 11 HIV-positive patients with no neurological problems (50[44-59] years, 82% males) and 12 HIV-negative controls (39[29-46] years, 75% males) were retrospectively analysed. Brain MRI protocol included axial T1, FLAIR and diffusion weighted imaging (DWI). All scans were processed by in-house semi-automatic procedures embedding segmentation of grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) on T1-weighted images, computation of brain tissues binary masks, DWI motion correction and DWI coregistration to T1. The Apparent Diffusion Coefficient (ADC) was fitted voxel-wise using a mono-exponential equation, and the resulting ADC maps were restricted to GM, WM, and CSF by using pertinent binary masks. The FLAIR scans were intensity normalized using the whole GM as an internal reference. The average GM intensity of the FLAIR signal (m) was calculated, and 1000/m was chosen as the global modulating factor and applied to the raw image values for intensity normalization.3 The volume of whole brain, WM, GM, and CSF was computed based on the binary tissues masks resulting from T1-weighted scan segmentation, after possible manual editing to correct for inaccuracies.Results
There was no statistically significant difference in the volumes of whole brain and individual brain tissues among the groups studied; however, the volume of GM in the HIV-ESC showed much larger dispersion than the other groups (Figure 2). A significant negative correlation (Spearman ρ = -0.73, p = 0.007) between GM volume and time from HIV infection to MRI was found in the HIV-ESC patient group. The median FLAIR signal intensity was significantly higher in the WM of HIV-ESC and HIV-ENC patients, than in the other two groups (Figure 3); no differences were found in GM and CSF. Both HIV-ESC and HIV-ENC patients showed significantly higher ADC values as compared with HIV and CTRL, in the whole brain, in GM and in WM (Figure 4). No statistically significant differences were found in CSF among the four groups. The HIV-ESC and HIV-ENC patient groups did not differ significantly from each other in any of the brain tissues, and neither did the HIV from the CTRL group.Discussion
At onset of CSF escape, WM hyperintensity can involve both supra and infra tentorial regions (periventricular (PV) or other areas, including cerebellum, corpus callosum (CC), corticospinal tracts (CS)). Deep White Matter (DWM) vasogenic edema is seen as an intense and extensive DWM FLAIR signal alteration. This edema isn’t associated to DWI changes/ADC restriction due to its extracellular location. This edema can be associated or not with mass effect depending on the degree of edema and underlying atrophy of patient’s brain. Edema determines a cortical sulcal effacement, PV ependima’s swelling with reduction of ventricular volume and intense and hyperintensity in T2 images. Due to extensive brain swelling, subarachnoidal venular stasis is appreciable and it is shown as contrast enhancement in cortical sulci – similar to the one that is found in slightly meningitis. This pattern changes during resolution treated escape with progressive reduction of all components of WM swelling/vasogenic (extracellular) edema until complete disappearance of signal alteration during follow up. This evolution can reach the complete resolution of the findings. Typically, underlying atrophy may re-emerge. Of note, the improvement of signal alteration starts at the same time of the beginning of clinical stabilization. This happens in a progressive way but more slowly that the clinical resolution with a sort of mismatch of clinico-radiological features.Conclusion
Although HIV-ESC and HIV-ENC occur in different clinical setting, they may show similar MRI measures. The low GM volumes in HIV-ESC with a long history of infection may be the legacy of a previous irreversible neuronal damage.Acknowledgements
No acknowledgement found.References
1. Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr HIV/AIDS Rep. 2015;12(2):280-288.
2. Mastrangelo A, Turrini F, de Zan V, Caccia R, Gerevini S, Cinque P. Symptomatic cerebrospinal fluid escape. AIDS. 2019;33 Suppl 2:S159-S169.
3. Huppertz HJ, Wagner J, Weber B, House P, Urbach H. Automated quantitative FLAIR analysis in hippocampal sclerosis. Epilepsy Research. 2011;97(1):146-156.
Figures
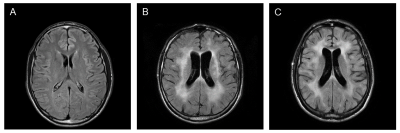
Figure 1. Representative FLAIR
MRI scans from the three HIV groups under study. (A) HIV-positive
patient with no neurological problems (HIV), (B) cART-untreated HIV
encephalitis (HIV-ENC) patient and (C) HIV CSF Escape (HIV-ESC) patient.
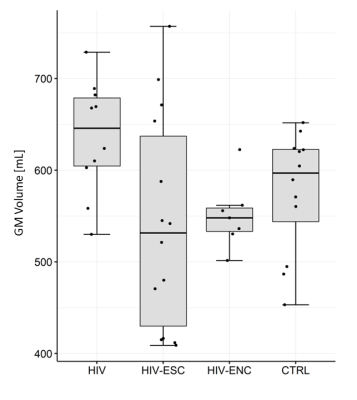
Figure 2. Distribution of GM
volume in HIV-ESC patients, HIV-ENC patients, HIV-positive patients with no
neurological problems and HIV-negative controls.
Abbreviations: GM = grey matter, HIV-ESC = HIV
CSF Escape, HIV-ENC = cART-untreated HIV encephalitis, HIV = HIV-positive
patients without neurological manifestations, CTRL = HIV-negative controls.
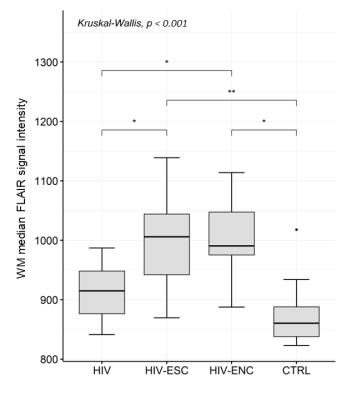
Figure 3. Distribution of WM median
FLAIR signal intensity in HIV-ESC patients, HIV-ENC patients, HIV-positive
patients with no neurological problems and HIV-negative controls. * indicates
the level of significance (** p < 0.001, * p < 0.05, (*) p < 0.06).
Abbreviations: FLAIR =
Fluid-Attenuated Inversion Recovery, WM = white matter, HIV-ESC = HIV CSF
Escape, HIV-ENC = cART-untreated HIV encephalitis, HIV = HIV-positive patients
without neurological manifestations, CTRL = HIV-negative controls.
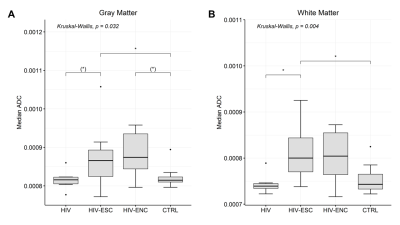
Figure 4. Distribution of GM (A)
and WM (B) ADC values in HIV-ESC patients, HIV-ENC patients,
HIV-positive patients with no neurological problems and HIV-negative controls. *
indicates the level of significance (** p < 0.001, * p < 0.05, (*) p <
0.06).
Abbreviations: ADC =
apparent diffusion coefficient, GM = grey matter, WM = white matter, HIV-ESC =
HIV CSF Escape, HIV-ENC = cART-untreated HIV encephalitis, HIV = HIV-positive
patients without neurological manifestations, CTRL = HIV-negative controls.
DOI: https://doi.org/10.58530/2023/2077