2064
Radiomics of MRI Based on Zoomit DWI in Predicting Clinically Significant Prostate Cancer: A Towards Step of a Non-invasive Auxiliary Tool1Department of radiology, First Affiliated Hospital of Soochow University, Suzhou, China, 2MR Scientific Marketing, Siemens Healthineers Ltd, Shanghai, China
Synopsis
Keywords: Prostate, Diffusion/other diffusion imaging techniques, Zoomit DWI; Resolve DWI;
Radiomics models based on ZOOMit DWI had better accuracy in the diagnosis of PCa and csPCa compared with those based on RESOLVE DWI technology, and was promising as a powerful non-invasive auxiliary tool to improve the diagnostic performance of PI-RADS of radiologists with different clinical experience.Introduction
Precise diagnosis of clinically significant prostate cancer (csPCa) in the early phase is important for the initiation of treatment and can improve outcomes [1]. Multiparametric magnetic resonance imaging (mpMRI) is increasingly being utilized for the diagnosis and the risk stratification of PCa [2-5]. As an important part of mpMRI, diffusion weighted imaging (DWI) sequence, particularly with ultra-high b-values (≥ 1000 s/mm²), can assess the restrictions information of water molecules in the random Brownian motion, which often occurs in areas of malignant lesions [6]. The readout-segmented echo-planar imaging (rs-EPI), by which the resolve_DWI is accomplished, and parallel transmit EPI (ptx-EPI), by which the zoomit_DWI is accomplished, can enhance image quality compared to traditional single shot EPI (ss-EPI) technique [7-9]. A recent radiomics study has reported that radiomics models based on the zoomit_DWI sequence had higher diagnostic accuracy for PCa than those based on the conventional DWI sequence [10]. However, as far as we know, no research has compared the performance of radiomic features based on resolve and zoomit technologies for the detection of PCa and csPCa, and it is yet unclear whether the radiomics models based on zoomit_DWI can improve the radiologists’ performance with various clinical experiences in the diagnoses of PCa and csPCa. Therefore, we aimed to evaluate the performance of the radiomics models based on zoomit_DWI in the diagnosis of csPCa compared with resolve_DWI, and explore the potential value of radiomics model to improve the performance of radiologists for the Prostate Imaging Reporting and Data System (PI-RADS) assessment.Methods
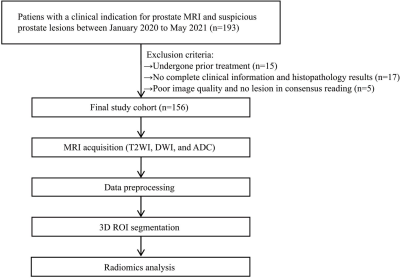
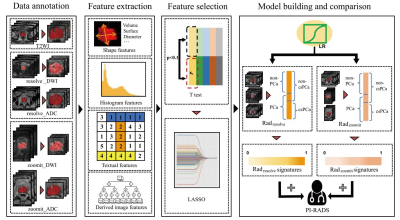
A total of 156 patients were included in this retrospective study. PI-RADS assessments of bpMRI were performed by the junior, senior, and expert-level radiologists of prostate MRI, respectively. The radiomics models (i.e., Radresolve and Radzoomit) of bpMRIs including resolve_DWI and zoomit_DWI were built, respectively. Then 3 mixed radiomics models integrating Radzoomit and the PI-RADS assessments of those 3 radiologists were built, respectively. Comparisons of diagnostic performance were performed between the radiomics models of zoomit_DWI and those of resolve_DWI, between the radiomics models of bpMRI, as well as between the mixed radiomics models and PI-RADS assessments.Results
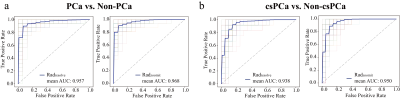
Radzoomit showed significantly higher AUCs than Radresolve. Both Radzoomit and Radresolve presented significantly higher AUCs than the PI-RADS assessments. Additionally, the mixed model integrating Radzoomit and the PI-RADS assessments of each of the junior and senior were higher than and comparable to the PI-RADS assessments of expert for PCa and csPCa diagnoses, respectively.Conclusions
The radiomics models of zoomit_DWI showed better diagnostic performance for PCa and csPCa than those of resolve_DWI. The bpMRI radiomics model is promising as a powerful non-invasive auxiliary tool to improve the diagnostic performance of PI-RADS of radiologists with different clinical experiences.Acknowledgements
The authors thank all those who helped us during the writing of this research. We also thank the Department of Urology and Pathology of the hospitals for their valuable help and feedback.References
1. van den Bergh RCN, Loeb S, Roobol MJ. Impact of Early Diagnosis of Prostate Cancer on Survival Outcomes. Eur Urol Focus. 2015;1:137-46. doi:10.1016/j.euf.2015.01.002.
2. Drost FJH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019:259. doi:10.1002/14651858.CD012663.pub2.
3. Bjurlin MA, Meng XS, Le Nobin J, Wysock JS, Lepor H, Rosenkrantz AB, et al. Optimization of Prostate Biopsy: the Role of Magnetic Resonance Imaging Targeted Biopsy in Detection, Localization and Risk Assessment. J Urol. 2014;192:648-58. doi:10.1016/j.juro.2014.03.117.
4. Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol. 2015;67:1112-21. doi:10.1016/j.eururo.2014.10.033.
5. Thompson JE, van Leeuwen PJ, Moses D, Shnier R, Brenner P, Delprado W, et al. The Diagnostic Performance of Multiparametric Magnetic Resonance Imaging to Detect Significant Prostate Cancer. J Urol. 2016;195:1428-35. doi:10.1016/j.juro.2015.10.140.
6. Padhani AR, Liu G, Mu-Koh D, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. Neoplasia. 2009;11:102-25. doi:10.1593/neo.81328.
7. Thian YL, Xie WY, Porter DA, Ang BW. Readout-segmented Echo-planar Imaging for Diffusion-weighted Imaging in the Pelvis at 3T-A Feasibility Study. Acad Radiol. 2014;21:531-7. doi:10.1016/j.acra.2014.01.005.
8. Klingebiel M, Ullrich T, Quentin M, Bonekamp D, Aissa J, Mally D, et al. Advanced diffusion weighted imaging of the prostate: Comparison of readout-segmented multi-shot, parallel-transmit and single-shot echo-planar imaging. European Journal of Radiology. 2020;130:8. doi:10.1016/j.ejrad.2020.109161.
9. Thierfelder KM, Scherr MK, Notohamiprodjo M, Weiss J, Dietrich O, Mueller-Lisse UG, et al. Diffusion-weighted MRI of the prostate: advantages of Zoomed EPI with parallel-transmit-accelerated 2D-selective excitation imaging. Eur Radiol. 2014;24:3233-41. doi:10.1007/s00330-014-3347-y.
10. Hu L, Zhou DW, Fu CX, Benkert T, Jiang CY, Li RT, et al. Advanced zoomed diffusion-weighted imaging vs. full-field-of-view diffusion-weighted imaging in prostate cancer detection: a radiomic features study. Eur Radiol. 2021;31:1760-9. doi:10.1007/s00330-020-07227-4.
Figures
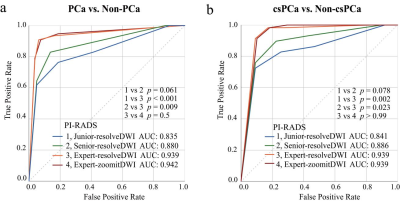
The ROC curves of bpMRI radiomics models for the classifications of PCa from non-PCa (a), and those of csPCa from non-csPCa (b) in the testing cohort
In each subfigure of (a) and (b), the thin solid curves are the ROC curves of the 100 classification models, and the thick solid line is the ROC curve of the mean of the classification performance across these 100 classification models.
Note:
Radresolve, radiomics model based on the combination of resolve_DWI + resolve_ADC + T2WI;
Radzoomit, radiomics model based on the combination of zoomit_DWI + zoomit_ADC + T2WI.
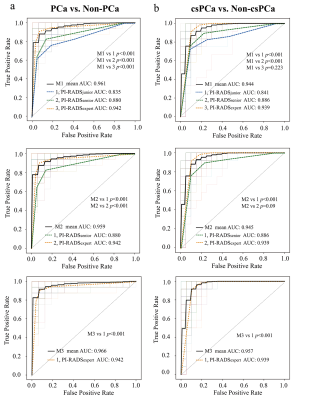
The ROC curves of mixed models for the diagnosis of PCa (a) and csPCa (b)
In each subfigure of (a) and (b), the thin solid curves are the ROC curves of the 100 classification models, and the thick solid line is the ROC curve of the mean of the classification performance across these 100 classification models, and the dotted curves are the ROC curves of radiologists with the junior, senior and expert.
Notes: M1, mixed model of Radzoomit + PI-RADSjunior;
M2, mixed model of Radzoomit + PI-RADSsenior;
M3, mixed model of Radzoomit + PI-RADSexpert;