2056
Feasibility of Prostate MR Fingerprinting and ADC Mapping in the quantitative characterization of malignant transition zone lesions1Department of Radiology, University of Michigan, Ann Arbor, MI, United States, 2Department of Diagnostic and Interventional Radiology, All Indi Institute of Medical Sciences, Jodhpur, India, 3Department of Urology, Case Western Reserve University, Cleveland, OH, United States, 4Department of Biostatistics, University of Michigan, Ann Arbor, MI, United States, 5Department of Radiology, Case Western Reserve University, Cleveland, OH, United States
Synopsis
Keywords: Prostate, Cancer
The purpose of this study is to assess the feasibility of the use of MR Fingerprinting combined with ADC mapping for characterizing prostate transition zone (TZ) cancers. This quantitative approach has shown promising results in differentiating prostate cancer from normal prostate tissue in peripheral and transition zones, and in separating low from intermediate/high grade tumors in the PZ. Our study aimed to confirm previous observations of quantitative differences in T1, T2 and ADC between cancers and normal appearing TZ, and to assess the utility of MRF-derived T1 and T2 mapping in distinguishing low grade lesions from high/intermediate-grade cancers in TZ.Introduction
Multiparametric prostate MRI (mp-MRI) using Prostate Imaging, Reporting, and Data System version 2 (PIRADS v2) is widely used in the qualitative diagnostic evaluation of prostate lesions1. However, there remains subjectivity and variability in lesion detection and characterization2. Further, differentiation of low-grade lesions which are indolent, from intermediate and high-grade lesions which have greater aggressiveness, is not typically possible. This current state leads to high biopsy rates, over-diagnosis, and over-treatment, and to systematic biopsy persisting as a norm3. Lesion characterization in the transition zone (TZ) is particularly challenging due to potential overlap between tumors and benign prostatic hyperplasia nodules. Apparent diffusion coefficient (ADC) mapping is the only quantitative technique in the current protocol. While it has been shown to have potential for the evaluation of tumor aggressiveness and differentiation between cancer and prostatitis4, diffusion imaging is still used mostly qualitatively in the PIRADS framework. Magnetic resonance fingerprinting (MRF) allows simultaneous quantitative and accurate mapping of multiple tissue properties and has been most used for measurements of T1 and T2 relaxation times5. This technology has been applied in combination with ADC mapping for prostate lesion characterization in the peripheral and transition zones6-8. Using cognitively and in-bore targeted biopsies for obtaining pathological correlation, this approach has shown promising results in differentiating prostate cancer from normal prostate tissue in both PZ6,7 and TZ8, as well as in separating low grade from intermediate/high grade tumors in the PZ. Separation between tumor grade in the TZ has not been previously reported. The aim of this study is to verify prior observations in the TZ in a cohort of patients with targeted in-bore or fusion biopsy combined with systematic biopsy, and to evaluate if MRF and/or ADC can help distinguish low grade from high grade tumors in the TZ.Methods
In this IRB-approved, HIPAA-compliant, single institution study, 34 prostate MRI studies from 34 patients (mean age 64, range 46-76 years) were acquired on 3T scanners between December 2016 and October 2020. Acquisition settings for MRF were as per literature6-8 and included: FOV 400×400 mm2, TR 11–13 ms, flip angle 5–75° and resolution 1×1×5 mm3, whole prostate coverage, 39 seconds per slice. EPI based ADC mapping was obtained (b=50-1600 s/mm2). Lesions were identified via the PIRADS v2 system as per clinical routine. All patients had biopsy-proven (17 systematic transrectal biopsy and 17 targeted biopsies – 9 fusion, 7 in gantry and 1 cognitively targeted) malignant TZ lesions correlating to the PIRADS v2 identified lesions. Of the tumors, 15 were low-grade (Gleason 6) and 19 clinically significant (Gleason score ≥ 7) tumors. The images were analyzed retrospectively, and regions of interest (ROIs) were placed on the TZ tumors guided by the radiology reports, and on the contralateral normal-appearing TZ (NATZ) on T1, T2, and ADC maps. Whole lesion ROIs and small ROIs over a subjectively identified tumor center were drawn. One patient had no identifiable NATZ, due to extensive tumor in almost the entire TZ. Paired t-tests were used to evaluate both MRF and ADC for the differentiation of NATZ and tumors. Two sample t-tests were used to evaluate differences in T1, T2 and ADC between clinically significant (Grade Group 2 or Gleason score ≥7) tumors and low-grade tumors (Grade Group 1 or Gleason score 6). Multiple logistic regression was used to test the association of tumor grade (low vs high) with the combination of MR fingerprinting and ADC. A p-value < 0.05 was considered to be statistically significant.Results and Discussion
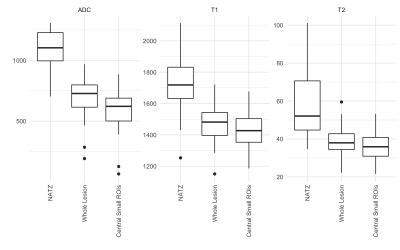
Representative relaxation time maps and ROIs in a cancerous lesion are shown in Figure 1. ADC, T1 and T2 were all lower in tumor as compared to NATZ (p<0.0001 for all, ΔADC=482x10-6 mm2/s, ΔT1=289 ms, ΔT2=23 ms for whole lesion ROIs, and ΔADC=381x10-6 mm2/s, ΔT1=250 ms, ΔT2=20 ms for central ROIs). Boxplots illustrating these differences are shown in Figure 2. This verifies prior reports that T1, T2 and ADC for tumor in the TZ are lower than normal appearing transition zone, despite the geographic heterogeneity observed in NATZ. T2 and ADC measurements did not help differentiate between tumor grades (p>>0.05). Measurements from T1 maps from whole tumor ROIs yielded a strong trend towards differentiating grade (p=0.06), though this did not reach our significance criteria. Mean ± SD in T1 maps were 1526 msec ± 98 in low grade tumors, and 1437 msec ± 125, in clinically significant tumors. This is concordant with the prior observation that T1 measurements are important in characterizing TZ tumors8. However, further work with more patients and carefully targeted biopsies or post-surgical radiological-pathological correlation is needed, to see if there is a true difference in T1 in low and high grade TZ tumors.Conclusion
MRF-based T1, and T2 combined with ADC maps show significantly lower values in tumors compared to NATZ, demonstrating their utility in the additional quantitative characterization of TZ prostate cancer. Whole lesion T1 measurements show a promising trend for differentiating low grade and intermediate/high grade tumors, and which requires further study.Acknowledgements
We receive research support from Siemens Healthineers, NIH grants R01CA208236 and R37CA263583.References
[1] Barentsz JO,Weinreb JC, Verma S, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol. 2016; 69:41–49.
[2] Greer, Matthew D., et al. "Interreader variability of prostate imaging reporting and data system version 2 in detecting and assessing prostate cancer lesions at prostate MRI." AJR. American journal of roentgenology (2019): 1.
[3] Litwin, Mark S., and Hung-Jui Tan. "The diagnosis and treatment of prostate cancer: a review." Jama 317.24 (2017): 2532-2542.
[4] Jung, Sung Il, et al. "Transition zone prostate cancer: incremental value of diffusion-weighted endorectal MR imaging in tumor detection and assessment of aggressiveness." Radiology 269.2 (2013): 493-503.
[5] Ma, Dan et al. “Magnetic resonance fingerprinting.” Nature vol. 495,7440 (2013): 187-92. doi:10.1038/nature11971
[6] Yu AC, Badve C, Ponsky LE, et al. Development of a combined MR fingerprinting and diffusion examination for prostate cancer. Radiology. 2017; 283:729–738.
[7] Panda, Ananya, et al. "Targeted biopsy validation of peripheral zone prostate cancer characterization with MR fingerprinting and diffusion mapping." Investigative radiology 54.8 (2019): 485.
[8] Panda, Ananya, et al. "MR fingerprinting and ADC mapping for characterization of lesions in the transition zone of the prostate gland." Radiology 292.3 (2019): 685.