2043
Evaluating liver fibrosis in chronic hepatitis B patients using multi-compartment restriction spectrum imaging1Department of Medical Imaging Center, Nanfang Hospital, Southern Medical University, Guangzhou, China, 2Shenzhen United Imaging Research Institute of Innovative Medical Equipment, Shenzhen, China, 3MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Liver, Diffusion/other diffusion imaging techniques
This study evaluated the feasibility of the restriction spectrum imaging (RSI) model in the characterization and staging of liver fibrosis. The results demonstrated that the four-compartment model emerged as the best option and the C4 derived from the four-compartment model outperformed conventional apparent diffusion coefficient (ADC) values. Therefore, the four-compartment RSI model could be a noninvasive and in vivo diagnostic technique in the evaluation of chronic hepatitis, which is conducive to treatment decisions and patient management.Introduction
Liver fibrosis is defined by the extracellular accumulation of collagens, proteoglycans, and other macromolecules as a result of chronic injury. The staging of liver fibrosis in these patients can help guide decisions about the need for specific antiviral medication [1,2]. Although liver biopsy is now the gold standard for determining liver pathology and fibrosis stage, it is an invasive operation with several drawbacks [3]. Therefore, non-invasive and reliable approaches for diagnosing and staging liver fibrosis are required. Restriction spectrum imaging (RSI) is a novel multicompartmental diffusion model that accounts for cellular geometry and compartmentalization by using a multi-shell diffusion acquisition and high b-values. The diffusion-weighted signal is represented by RSI as a linear combination of exponential decay, with unique decay curves corresponding to various tissue compartments. Because the apparent diffusion coefficient (ADC) value of each compartment is fixed, the change in diffusion signal across voxels is interpreted as a variation in the fraction of each tissue compartment comprising the overall diffusion signal [4]. The purpose of this study was to optimize the multicompartmental RSI models and apply them to evaluate liver fibrosis in patients with chronic hepatitis B.Methods
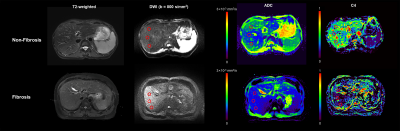
A cohort of 80 adult patients with liver fibrosis and 19 healthy subjects were included in this study and underwent magnetic resonance imaging (MRI) examination on a 3.0T scanner (uMR780, United Imaging Healthcare, Shanghai, China) with a 12-channel body coil. Diffusion-weighted imaging (DWI) was acquired using the echo planar imaging (EPI) sequence with the respiratory trigger in the axial plane (repetition time (TR) /echo time (TE): 4000 ms/78 ms, flip angle (FA): 90°, slice thickness: 5 mm, field of view (FOV): 300 × 356 mm2, matrix: 242 × 288, b-values: 0, 200, 400, 600, 800, 1,000, 2,000 s/mm2). The conventional ADC was computed by using images from two b-values, 0 and 800 s/mm2. The RSI model was defined by the following equation:$$$ S(b) = \sum_{i=1}^k{C_ie^{-bD_i}}$$$
where S(b) are signal intensities at different b-values, k is the number of tissue compartments, Ci describes the contribution of a particular compartment to the overall signal and Di is the compartmental ADC. To obtain optimal compartmental ADCs, multicompartmental models with 2-5 tissue compartments were fitted to DWI data.
For each subject, a total of six circular ROIs (mean area, 299.8mm2) were drawn within the right lobe of the liver on the central two continuous sections (three ROIs per section), avoiding large intrahepatic vessels and focal hepatic lesions. The left lobe and right liver dome were avoided as measurements could be unreliable from cardiac motion artifacts.
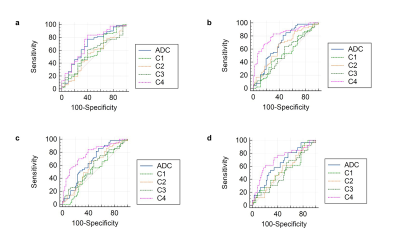
The Kruskal-Wallis rank sum tests were performed to evaluate whether the conventional ADC and compartmental signal-fraction derived from the optimal RSI model displayed significant differences among different fibrosis stages. Associations between DWI-derived parameters and histological fibrosis stages were evaluated by Spearman correlation coefficients. Besides, the five-point METAVIR score was dichotomized to generate four comparison groups: F0 versus F1/F2/F3/F4, F0/F1 versus F2/F3/F4, F0/F1/F2 versus F3/F4, and F0/F1/F2/F3 versus F4. Mann-Whitney U test was used to assess differences in compartmental signal-fraction and conventional ADC between different groups. Receiver operating characteristic analyses were performed to assess the diagnostic performance.
Results
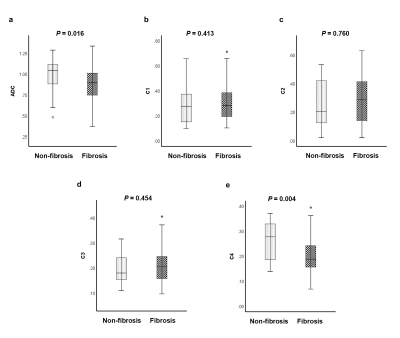
The lowest relatively Bayesian Information Criterion (BIC) was observed from the 4-compartment model, with optimal ADCs 7.31×10-10, 0.89×10-3, 3.00×10-3, >3×10-2 mm2/s. The conventional ADC and C4 were significantly lower in fibrotic tissues than in normal ones (P = 0.016 for conventional ADC and P = 0.004 for C4) (Figure 2). ADC and C4 were significantly different across all fibrosis stages (P = 0.026 for conventional ADC and P < 0.001 for C4). Both the conventional ADC and C4 exhibited a significant negative correlation with the fibrosis stage (ADC: r = -0.3238, P = 0.001; C4: r = -0.4931; P < 0.001). Compared with conventional ADC, the RSI model showed better diagnostic performance in characterizing and staging liver fibrosis and normal tissue (Figure 3).Discussion
Previous studies found a decrease of the ADC in fibrotic livers relative to controls [5], and a significantly negative correlation between the liver ADC and fibrosis stages [6, 7]. Our study obtained the same results as previous research. However, the mono-exponential model assumes Gaussian diffusion conditions and the conventional ADC only measures the overall diffusion level with a mix of different diffusion characteristics, such as restricted diffusion and rapid pseudo-diffusion arising from blood perfusion [8]. While liver fibrosis is a complex assemblage of collagen fibers, glycosaminoglycans, and proteoglycans [9], the impact of tissue structure on water diffusion cannot be adequately explained by a simple change in ADC. The RSI model is a multicompartmental approach to DWI that separates the overall diffusion signal into compartments. In this study, the C4 derived from the four-compartment model proved a superior voxel-level classifier for liver fibrosis than conventional ADC.Conclusion
This study showed the feasibility of the RSI model in the characterization and staging of liver fibrosis. The four-compartment model emerged as the best option and the C4 derived from the four-compartment model outperformed conventional ADC values. This advanced voxel-wise classifier could be a noninvasive and in vivo diagnostic technique in the evaluation of chronic hepatitis, which is conducive to treatment decisions and patient management.Acknowledgements
No acknowledgement found.References
1. Sohn, W., et al., Liver fibrosis scores and risk of liver‐related mortality in young adults with chronic hepatitis B: A cohort study. Journal of Viral Hepatitis, 2022. 29(1): p. 69-77.
2. Yang, F., et al., Noninvasive assessment of liver fibrosis for predicting acute-on-chronic liver failure in patients with chronic hepatitis B. Hepatology international, 2021. 15(3): p. 593-601.
3. Standish, R., et al., An appraisal of the histopathological assessment of liver fibrosis. Gut, 2006. 55(4): p. 569-578.
4. White, N.S., et al., Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer research, 2014. 74(17): p. 4638-4652.
5. Bakan, A.A., et al., Utility of diffusion-weighted imaging in the evaluation of liver fibrosis. European radiology, 2012. 22(3): p. 682-687.
6. Yang, L., et al., Staging liver fibrosis with DWI: is there an added value for diffusion kurtosis imaging? European radiology, 2018. 28(7): p. 3041-3049.
7. Annet, L., et al., Assessment of diffusion‐weighted MR imaging in liver fibrosis. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2007. 25(1): p. 122-128.
8. Chow, A.M., et al., Liver fibrosis: an intravoxel incoherent motion (IVIM) study. Journal of Magnetic Resonance Imaging, 2012. 36(1): p. 159-167.
9. Ramanujan, S., et al., Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophysical journal, 2002. 83(3): p. 1650-1660.
Figures