2026
A nomogram based on DKI, molecular and clinical risk factors for survival prediction and treatment decision-making in astrocytomas1First Hospital of Shanxi Medical University, Taiyuan, Shanxi Province, China, China, 2LinFen People's Hospital, Linfen, Shanxi Province, China, China
Synopsis
Keywords: Data Analysis, Tumor, diffusion kurtosis imaging, nomogram, astrocytoma, overall survival, treatment
To establish a nomogram by integrating DKI, molecular and clinical risk factors for personalized OS estimation in astrocytomas, and explore the nomogram-based treatment benefits. Astrocytomas are the most common brain tumors with poor prognoses. The detection of risk factors may aid in individualizing therapeutic plans and improve survival. It is concluded the nomogram based on DKI, molecular and clinical risk factors could achieve the individualized OS estimation of astrocytomas with excellent performance. For high-risk patients, surgery plus chemo and/or radiotherapy were recommended; while for low-risk patients, additional chemo and/or radiotherapy did not increase survival benefits.Background or Purpose
Astrocytomas represent the most common brain tumors with poor prognoses. The detection of useful risk factors may aid in individualizing therapeutic plans and prolong the survival of astrocytomas. Many factors could predict the prognosis of astrocytomas. Molecular classifications, which include isocitrate dehydrogenase (IDH), oxygen 6-methylguanine-DNA methyltransferase promoter (MGMT), and telomerase reverse transcriptase (TERT), have proven to be more precise for prognostic judgment [1]. Diffusion kurtosis imaging (DKI) can accurately describe the non-Gaussian distribution of water molecules, reflecting the cellular density and tissue heterogeneity of the brain parenchyma [2]. The parameters include mean kurtosis (MK), axial kurtosis, and fractional anisotropy (FA). Previous studies have proved the prognostic value of DKI in astrocytomas and MK was the optimal predictive parameter [3]. In clinical practice, a more comprehensive risk stratification model which combines DKI, molecular, and clinical factors may provide a better way for survival prediction of astrocytomas. Hence, we aimed to investigate the incremental value of constructing a nomogram based on DKI, molecular, and clinical risk factors for risk stratification of astrocytomas; and explore the value of nomogram scores in treatment selections.Methods
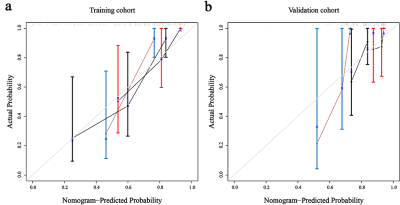
Sixty-four patients with clinical and molecular information were retrospectively analyzed. The DKI parameters were calculated based on the enhanced lesions on contrast-enhanced T1WI. A nomogram was constructed based on the risk factors identified by the univariate and multivariate Cox analysis. Calibration curves and ROC analysis were generated to assess the calibrating ability and the discrimination performance. Kaplan-Meier survival curves were generated and treatment benefits status was plotted according to the nomogram scores.Results
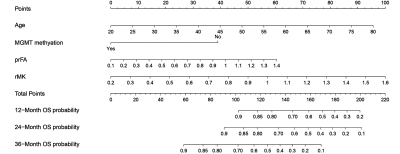
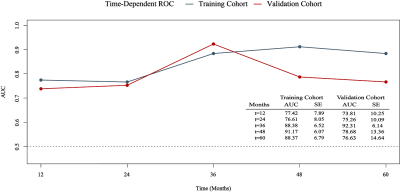
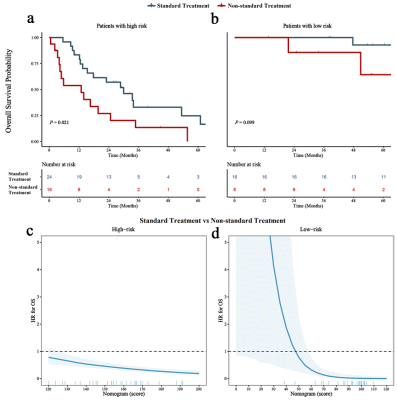
Relative MK (rMK), perilesional relative FA (prFA), age, and MGMT promoter methylation (MGMTmet) (P < 0.05) were prognostic factors of astrocytomas. The nomogram displayed favorable calibration capacity; Area under the curve at 1-, 2-, and 3-year in the training and validation cohorts were 0.774, 0.766, 0.883, 0.738, 0.752, and 0.923, respectively. Patients were classified into high-risk and low-risk subgroups by the best cut-off value of nomogram scores (110). Surgery plus chemo and/or radiotherapy prominently increased survival benefits for all nomogram scores compared with surgery only in the high-risk subgroup [Hazard Ratio (HR): 0.433, 95% Confidence Interval (CI), 0.208–0.901]; however, in the low-risk subgroup, there existed differences between surgery plus chemo and/or radiotherapy and surgery only (HR: 0.168, 95% CI, 0.015–1.867).Discussion
Previous studies have investigated the prognostic value of DKI in gliomas [3-6]. Our study further assessed the value of DKI in individualized survival prediction of astrocytomas by constructing a nomogram. Calibration plots demonstrated good consistency between the predictive and observational possibility in the training and validation cohorts. The AUC for predicting the 3-year survival reached 0.923, which is higher than that of the previous models (AUC = 0.841) [3]. More importantly, nomogram-based treatment benefits were also assessed.The present study identified rMK, prFA, age, and MGMTmet as prognostic factors of astrocytomas. Consistent with the previous studies, we proved that higher rMK values indicated shorter lifespans. MK reflects the complexity of tumor microstructure, and greater MK values were correlated with a higher degree of complexity within tumors [2, 3]. Astrocytomas with higher tumor heterogeneity exhibit increased vascular proliferation, more nuclear atypia, and greater cellularity. Thus, greater MK values represent a poorer prognosis of astrocytomas. We discovered that a higher value of prFA reflected a worse outcome. This might be because high-grade astrocytomas exhibit more arrangement patterns of tumor tissues, increasing the anisotropy of water molecule diffusions within the peritumoral edema region [7, 8]. The present study revealed that the OS of astrocytomas worsened with increasing age, the reason might be that the molecular characteristics of elderly patients were more aggressive than those of young patients [9]. The 2016 classification of central nervous system cancers first recognized MGMT as an important genetic hallmark for astrocytomas, and MGMTmet astrocytomas could benefit more from chemo-radiotherapy than unmethylated ones [10, 11]. Our results also proved that patients presenting MGMTmet had longer survival time.
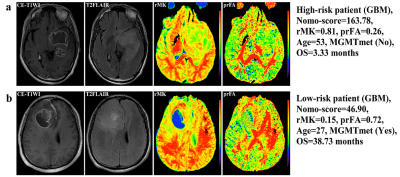
Additionally, our study indicated different treatment options for high-risk and low-risk subgroups. Patients with surgery plus chemo and/or radiotherapy might survive longer than those receiving only surgery in the high-risk subgroup, which is consistent with the National Comprehensive Cancer Network (NCCN) guidelines [12]. In the low-risk subgroup, however, the survival benefit of additional chemo and/or radiotherapy did not increase. This could be explained by the NCCN guidelines, which indicate that not all low-grade gliomas need adjuvant chemo and/or radiotherapy [12]. These results are important for the realization of individualized treatment strategies, which in turn, confirmed the practicability and reliability of the present nomogram.
Conclusions
rMK, prFA, age, and MGMTmet were prognostic biomarkers for astrocytomas. A nomogram incorporating these 4 prognostic factors exhibited excellent performance for personalized OS estimation. Further, surgery plus chemo and/or radiotherapy were recommended for high-risk patients, while the survival benefits of additional chemo and/or radiotherapy did not increase in the low-risk subgroup.Acknowledgements
No acknowledgement found.References
1. Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231-1251
2. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432-1440
3. Zhang J, Jiang J, Zhao L et al (2019) Survival prediction of high-grade glioma patients with diffusion kurtosis imaging. Am J Transl Res 11:3680-3688
4. Wang X, Gao W, Li F, Shi W, Li H, Zeng Q (2019) Diffusion kurtosis imaging as an imaging biomarker for predicting prognosis of the patients with high-grade gliomas. Magnetic Resonance Imaging 63:131-136
5. Wang X, Li F, Wang D, Zeng Q (2020) Diffusion kurtosis imaging combined with molecular markers as a comprehensive approach to predict overall survival in patients with gliomas. European Journal of Radiology 128:108985
6. Hempel JM, Brendle C, Bender B et al (2019) Diffusion kurtosis imaging histogram parameter metrics predicting survival in integrated molecular subtypes of diffuse glioma: An observational cohort study. European Journal of Radiology 112:144-152
7. Saksena S, Jain R, Narang J et al (2010) Predicting survival in glioblastomas using diffusion tensor imaging metrics. J Magn Reson Imaging 32:788-795
8. Huber T, Bette S, Wiestler B et al (2016) Fractional Anisotropy Correlates with Overall Survival in Glioblastoma. World Neurosurg 95:525-534.e521
9. Krigers A, Demetz M, Thomé C et al (2021). Age is associated with unfavorable neuropathological and radiological features and poor outcome in patients with WHO grade 2 and 3 gliomas. Scientific reports 11(1): 17380
10. Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803-820
11. Delgado-López PD, Corrales-García EM (2016) Survival in glioblastoma: a review on the impact of treatment modalities. Clinical & Translational Oncology 18:1062-1071
12. Nabors LB, Portnow J, Ahluwalia M et al (2020) Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 18:1537-1570
Figures