2021
Visualization of Perivascular Spaces using an Optimized 3D-TSE Sequence with Reduced Flip Angle at 7T1NINDS/LFMI, National Institutes of Health, Bethesda, MD, United States, 2NIMH/SSCC, National Institutes of Health, Bethesda, MD, United States, 3NINDS/NMRF, National Institutes of Health, Bethesda, MD, United States
Synopsis
Keywords: Visualization, Neurofluids, CSF
Perivascular spaces (PVS) dilatation has been recently linked to aging and neurodegenerative diseases. T2-weighted ultra-high field MRI allows to image the PVS burden at high contrast and spatial resolution non-invasively. However, most of sub-voxel sized PVS cannot be resolved due to partial volume effects and remaining signal from blood and tissue. High-resolution 3D-TSE with reduced flip angle allowed the visualization of PVS with a very high CSF-to-tissue ratio of ~37:1 at 7T.Introduction
Perivascular spaces (PVS) are fluid-filled spaces surrounding cerebral arteries and veins that are believed to exchange with interstitial fluid (ISF) and cerebrospinal fluid (CSF). In addition to playing an essential role in CSF turnover, recent studies have also revealed that PVS dilatation is associated with aging and neurodegenerative diseases1,2. Heavily T2-weighted MRI has been proven to be useful for detecting PVS as a potential biomarker for neurological disorders non-invasively3. Ultra-high field MRI has enabled the visualization of PVS with very high contrast and spatial resolution4-10. Although the emergence of 7T scanners has increased the capability of detecting sub-millimeter PVS, most remain below the achievable voxel size and thus, cannot be resolved due to partial volume effects and remaining signal from surrounding tissue. In this work, we optimized a 3D-Turbo Spin Echo (TSE) sequence with reduced flip angle taking advantage of long CSF T211,12 to image PVS with negligible signal from tissues or blood at 7T.Methods
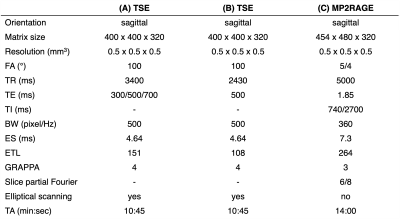
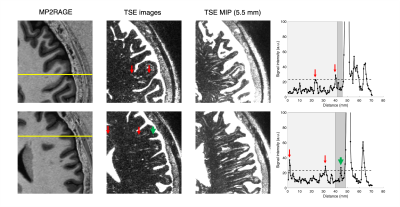
Three subjects (2 males and 1 female, age ~31-43 year old) were scanned on a 7T Terra Magnetom system (Siemens Healthineers, Germany) with a 1-Tx/32-Rx head array (Nova Medical, USA). High-resolution 3D-TSE scans were acquired with effective echo times (TE) of 300, 500 and 700 ms using imaging parameters shown in Table 1A. The total scan time was set to ~10 min to minimize motion artifacts. A reduced refocusing flip angle (FA) of 100° was chosen to minimize SAR and blurring. Additionally, TE 500 ms TSE images were acquired with a shorter echo train length (ETL) of 108 to further reduce blurring (Table 1B). High-resolution MP2RAGE anatomical images were acquired using the parameters listed in Table 1C. Numerical simulations were performed using an Extended Phase Graph (EPG) algorithm13 assuming relaxation times T1/T2 = 1220/46 ms for white matter (WM), T1/T2 = 2132/55 ms for gray matter (GM), T1/T2 = 2587/68 ms for blood and T1/T2 = 4329/2000 ms for CSF14-16. 3D-TSE and MP2RAGE images were aligned using imregister MATLAB affine registration tool. Four ROIs of variable sizes were manually selected and averaged across three different slices in the ventricles for CSF, WM, and deep GM regions. The WM and GM ROIs were placed in regions close to CSF ROIs to minimize contrast variations due to non-uniform flip angle distribution. CSF-to-tissue ratio was determined with respect to WM and GM ROIs. TSE images were visualized with maximum intensity projections (MIPs). Contrast-to-noise ratio (CNR) was calculated by the difference between mean CSF and tissue signal divided by the mean intensity of the noise ROIs outside the brain. The intensity threshold for PVS detection was set to two times the standard deviation above the mean of WM ROI signal at 500 ms TE.Results
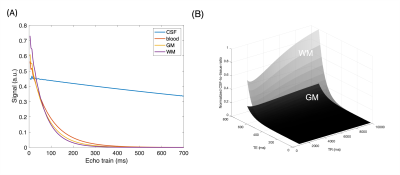
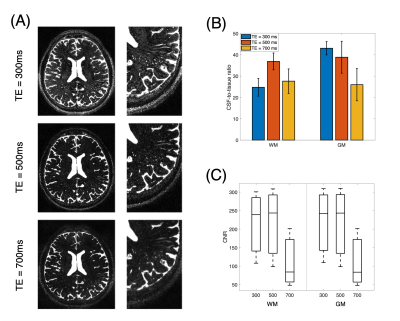
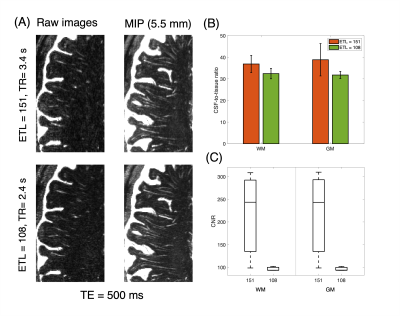
Figure 1A shows CSF, blood, GM, and WM simulated echo signal evolution with imaging parameters listed Table 1A. The plot shows that WM/GM/blood signal can be reduced to < 0.05% of the equilibrium values by employing TE values greater than 500 ms. CSF-to-tissue ratio is expected to be maximized at very long TR and TE when ignoring noise (Figure 1B). Figure 2A shows images acquired at different TE values. Longer TEs (500 & 700 ms) show the CSF filled PVS against a lower background signal since the WM and GM signals are effectively suppressed. However, longer TEs introduce some blurring of the small PVS. The measured CSF-to-WM ratio was highest ~37:1 at TE 500 ms and the CSF-to-GM ratio similar (Figure 2B). The mean CSF-to-tissue CNR was found to be ~244 (Figure 2C). Figure 3A shows that the use of a protocol with a shorter ETL (Table 1B) improved the overall image sharpness allowing better depiction of PVS. CSF-to-tissue ratio and CNR where slightly reduced with the shorter ETL protocol (Figure 3B & 3C) compared to longer ETL protocols due to the use of a shorter TR. Figure 4 shows WM and cortical PVS in TSE images acquired with imaging parameters listed in Table 1B. The intensity threshold used for PVS detection corresponds to 1/20 of the CSF signal in the ventricles. Therefore, the current imaging sequence could detect PVS volumes as small as ~6 nL.Discussion
3D-TSE MRI with long TE (~500 ms) and reduced flip angle allowed acquisition of high-resolution CSF images without residual signal from other tissues. This protocol enabled visualization of small PVS with a CSF-to-tissue ratio of ~37:1 at 7T. At this contrast, it was estimated that CSF fractions could be detected at a few nanoliters per voxel. Further increase in PVS detection sensitivity will be possible by increasing the TR. In this case, accelerated acquisition methods such as compressed sensing17 or motion correction18 may be required to reduce motion related artifacts due to long scan times. Moreover, more uniform contrast could be achieved throughout the whole brain using tailored or universal RF pulses with parallel transmission19-21. Finally, future work will assess the efficiency of this sequence with different processing approaches to improve the quantification of sub-voxel sized PVS at 7T.Conclusion
PVS burden can be imaged non-invasively at very high resolution and sensitivity using 3D-TSE MRI with reduced flip angle at 7T. This could help for early detection of neurodegenerative diseases.Acknowledgements
No acknowledgement found.References
1. Wardlaw JM, Smith EE, Biessels GJ, et al. Standards for Reporting Vascular changes on neuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013 Aug;12(8):822-38.
2. Ramirez J, Berezuk C, McNeely AA, et al. Imaging the Perivascular Space as a Potential Biomarker of Neurovascular and Neurodegenerative Diseases. Cell Mol Neurobiol. 2016 Mar;36(2):289-99.
3. Tsitouridis I, Papaioannou S, Arvaniti M, et al. Enhancement of virchow-robin spaces. An MRI evaluation. Neuroradiol J. 2009 Jan 20;21(6):773-9.
4. Bouvy WH, Biessels GJ, Kuijf HJ, et al. Visualization of perivascular spaces and perforating arteries with 7T magnetic resonance imaging. Invest Radiol. 2014 May;49(5):307-13.
5. Bouvy WH, Zwanenburg JJM, Reinink R, et al. Perivascular spaces on 7 Tesla brain MRI are related to markers of small vessel disease but not to age or cardiovascular risk factors. J Cereb Blood Flow Metab. 2016 Oct;36(10):1708-1717.
6. Spijkerman JM, Zwanenburg JJM, Bouvy WH, et al. Automatic quantification of perivascular spaces in T2-weighted images at 7 T MRI. Cereb Circ Cogn Behav. 2022 Apr 5;3:100142.
7. Cai K, Tain R, Das S, et al. The feasibility of quantitative MRI of perivascular spaces at 7T. J Neurosci Methods. 2015 Dec 30;256:151-6.
8. Zong X, Park SH, Shen D, Lin W. Visualization of perivascular spaces in the human brain at 7T: sequence optimization and morphology characterization. Neuroimage. 2016 Jan 15;125:895-902.
9. Feldman RE, Rutland JW, Fields MC, et al. Quantification of perivascular spaces at 7T: A potential MRI biomarker for epilepsy. Seizure. 2018 Jan;54:11-18.
10. Zong X, Lian C, Jimenez J, et al. Morphology of perivascular spaces and enclosed blood vessels in young to middle-aged healthy adults at 7T: Dependences on age, brain region, and breathing gas. Neuroimage. 2020 Sep;218:116978.
11. Gao KC, Nair G, Cortese IC, et al. Sub-millimeter imaging of brain-free water for rapid volume assessment in atrophic brains. Neuroimage. 2014 Oct 15;100:370-8.
12. Daoust A, Dodd S, Nair G, et al. Transverse relaxation of cerebrospinal fluid depends on glucose concentration. Magn Reson Imaging. 2017 Dec;44:72-81.
13. Hennig J, Weigel M, Scheffler K. Multiecho sequences with variable refocusing flip angles: optimization of signal behavior using smooth transitions between pseudo steady states (TRAPS). Magn Reson Med. 2003 Mar;49(3):527-35.
14. Visser F, Zwanenburg JJ, Hoogduin. High-resolution magnetization-prepared 3D-FLAIR imaging at 7.0 Tesla. Magn Reson Med. 2010 Jul;64(1):194-202.
15. Zhang X, Petersen ET, Ghariq E, et al. In vivo blood T1 measurements at 1.5T, 3T, and 7T. In vivo blood T1 measurements. Magn Reson Med. 2013;70:1082-1086.
16. Krishnamurthy LC, Liu P, Xu F, et al. Dependence of blood T2 on oxygenation at 7T: in vitro calibration and in vivo application: blood T2 at 7 T. Magn Reson Med. 2014;71:2035-2042.
17. Hirschler L, Runderkamp BA, Franklin SL. The driving force of glymphatics: influence of the cardiac cycle on CSF-mobility in perivascular spaces in humans. Proceedings ISMRM 2020.
18. Zong X, Nanavati S, Hung SC, et al. Effects of motion and retrospective motion correction on the visualization and quantification of perivascular spaces in ultrahigh resolution T2-weighted images at 7T. Magn Reson Med. 2021 Oct;86(4):1944-1955.
19. Van Damme L, Mauconduit F, Chambrion T, et al. Universal nonselective excitation and refocusing pulses with improved robustness to off-resonance for Magnetic Resonance Imaging at 7 Tesla with parallel transmission. Magn Reson Med. 2021 Feb;85(2):678-693.
20. Gras V, Mauconduit F, Vignaud A, et al. Design of universal parallel-transmit refocusing kT -point pulses and application to 3D T2-weighted imaging at 7T. Magn Reson Med. 2018 Jul;80(1):53-65.
21. Ehses P, Hirschler L, Pracht ED. Calibration free parallel transmission for improved for improved CSF mobility imaging at 7T. Proceedings ISMRM 2022.
Figures