2018
Distortion-free diffusion imaging using single-shot diffusion-prepared TSE sequence with spiral-ring readouts and magnitude stabilizers
Zhixing Wang1, Xiaozhi Cao2, Kun Qing3, Xue Feng1, John P. Mugler4, and Craig H. Meyer1,4
1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Department of Radiology, Stanford University, Stanford, CA, United States, 3Radiation Oncology, City of Hope, Duarte, CA, United States, 4Radiology & Medical Imaging, University of Virginia, Charlottesville, VA, United States
1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Department of Radiology, Stanford University, Stanford, CA, United States, 3Radiation Oncology, City of Hope, Duarte, CA, United States, 4Radiology & Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
Keywords: Data Acquisition, Data Acquisition
This study provides an alternative approach to conventional diffusion-weighted (DW) EPI-based sequences. A 2D single-shot (SS) diffusion-prepared (DP) turbo-spin-echo (TSE) sequence, combined with spiral-ring trajectories and magnitude stabilizers, dubbed “SS-DP-SPRING TSE”, was developed for distortion- and motion artifact-free diffusion imaging. Compared to a SS-DW-EPI sequence, this method is less sensitive to B0-inhomogeneity and thus provides DW-images with improved geometric fidelity.Introduction:
Diffusion-weighted pulse sequences are routine MRI protocols for neurological and oncological imaging, including, but not limited to, acute stroke and tumors.1,2 The combination of single-shot spin-echo acquisition and an EPI-readout enables fast diffusion-weighted imaging with minimal motion artifacts. However, the long EPI-readout duration presents challenges for imperfections, such as B0-inhomogenity induced geometric distortions and ghosting artifacts, thus limiting its use for some clinical applications (e.g., tumor delineation for radiation therapy). Multi-shot (MS) DW-EPI is a means to improve geometric fidelity3, yet shot-to-shot phase inconsistency caused by physiological motion (e.g., cardiac pulsation) and system imperfections can lead to substantial signal dropouts and artifacts, which require additional motion information to suppress and cannot always be easily corrected by a phase-correction method.4,5TSE acquisition6 uses a series of refocusing RF pulses that split long sampling trajectories (e.g., a spiral readout) into small portions of k-space coverage to suppress effects of B0-inhomogenity. Researchers have developed sampling strategies including spiral-ring TSE for fast PD/T2-weighted imaging.7-8 However, incorporating diffusion gradients into a TSE acquisition is not straightforward because of the Carr-Purcell-Meiboom-Gill (CPMG) condition.9 In this work, we utilize a diffusion-prepared approach10,11 combined with spiral-ring TSE imaging for single-shot diffusion imaging. Additionally, we include gradient stabilizers to correct for magnitude inconsistency associated with the preparation module.
Methods:
A schematic of the proposed SS-DP-SPRING TSE sequence is depicted in Figure 1. The diffusion-prepared (DP) module consisted of Stejskal–Tanner monopolar diffusion-gradients and one magnitude stabilizer (~4π dephasing) along the slice-select direction, followed by a 90° tip-up RF pulse that flips the diffusion-encoded signal back to the longitudinal axis. Large spoiler gradients (~2X larger than stabilizers) were added immediately after the diffusion-prepared module and before the following TSE acquisition. Rephasing and dephasing gradients were inserted before and after each echo during the readout to form the echo and distribute the magnetization, respectively. The added stabilizers offered the benefit of converting the magnitude-modulated signal loss into a phase-modulated problem9, at the cost of 50% SNR loss. We further adopted a single-shot acquisition approach, so there is no need of additional phase-correction which may not completely resolve phase inconsistencies among shots.The central spiral-in-out ring was placed at the first echo of the TSE acquisition to obtain a short TE (~11 ms). Ten spiral-rings with linearly decreasing sampling density, from 1 to 0.2, were designed for 1.25 mm2 isotropic in-plane resolution, with a total TSE acquisition time of 110 ms. The duration of diffusion gradients was 12 ms with 71 mT/m amplitude for an estimated b-value of 750 s/mm2, yielding a total duration of 35 ms for the DP module. The slice-thickness of the sinc RF pulses used in the DP module was 1.5X larger than that of the TSE acquisition to mitigate flow-related and cross-talk artifacts. Other sequence parameters are given in Table 1.
To demonstrate the efficiency of stabilizers combined with the single-shot theme, we tested SS-DP-SPRING TSE without stabilizers and with stabilizers, and compared it to 2-shot DP-SPRING TSE with stabilizers. For image reconstruction, NUFFT12 and L1-ESPIRiT13 were performed on undersampled datasets.
Experiments were performed on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil array. For both phantom and healthy volunteer studies, multiple slices with 4-mm thickness (2-mm gap) were acquired using the proposed methods and SS-DW-EPI.
Results and Discussion:
Figure 2 shows how the slice-thickness of RF pulses in the preparation module, and the dephasing range of stabilizers, affect image quality. The diffusion gradient was off for all phantom images. Compared to the reference (without preparation module), image quality degrades when the slice-thickness is small or stabilizers are not large enough to uniformly distribute the phase, most likely due to the slice-overlap artifacts, although there are still minor residual artifacts for the proposed method.Figure 3 demonstrates the performance improvement for the proposed SS-DP-SPRING TSE with magnitude stabilizers (top) compared to that without stabilizers (middle) and for 2-shot DP-SPRING TSE with stabilizers (bottom). All images were reconstructed using NUFFT. Unpredictable severe bands of signal loss can be seen in Figure 3F-G from SS-DP-SPRING TSE without stabilizers when the diffusion gradient is on. When using 2-shot acquisition, artifacts and signal cancellation are obvious as shown in Figure 3L, because of cardiac pulsation, when the diffusion gradient is along head-foot direction. Image degradation attributed to magnitude or phase modulation is substantially reduced when applying stabilizers along with the single-shot theme.
Figure 4 shows examples of estimated ADC maps along three main diffusion directions and the mean ADC, acquired by SS-DP-SPRING TSE with magnitude stabilizers and L1-ESPIRiT reconstruction (top) and SS-DW-EPI (bottom). A T2-weighted Cartesian TSE image is used for anatomical reference. The proposed method shows similar ADC maps compared to the SS-DW-EPI counterpart, while distortion and signal pile-up artifacts are clearly seen in EPI images (e.g., red arrow). However, some slices from SS-DP-SPRING TSE show residual image artifacts (not shown here), likely due to the imperfect slice profile of RF pulses used in the DP module; future work is warranted to investigate this issue.
Conclusion:
We demonstrated a 2D single-shot diffusion-prepared TSE acquisition that utilized variable-density spiral-ring trajectories and magnitude stabilizers for distortion- and motion artifact-free diffusion-weighted brain imaging.Acknowledgements
No acknowledgement found.References
[1] Lövblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion‐weighted MR in patients with acute stroke. AJNR Am J Neuroradiol. 1998;19:1061–1066.[2] Tsien C, Cao Y, Chenevert T. Clinical applications for diffusion magnetic resonance imaging in radiotherapy. Semin Radiat Oncol. 2014;24:218–226.
[3] Holdsworth SJ, Skare S, Newbould RD, Guzmann R, Blevins NH, Bammer R. Readout‐segmented EPI for rapid high resolution diffusion imaging at 3T. Eur J Radiol. 2008;65:36–46.
[4] Skare S, Andersson JL. On the effects of gating in diffusion imaging of the brain using single shot EPI. Magn Reson Imaging. 2001;19:1125–1128.
[5] O’Halloran RL, Holdsworth S, Aksoy M, Bammer R. Model for the correction of motion‐induced phase errors in multishot diffusion‐ weighted‐MRI of the head: are cardiac‐motion‐induced phase errors reproducible from beat‐to‐beat? Magn Reson Med. 2012;68:430–440.
[6] Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823-833.
[7] Hennig J, Barghoorn A, Zhang S, Zaitsev M. Single shot spiral TSE with annulated segmentation. Magn Reson Med. 2022;88:651-662.
[8] Wang Z, Allen SP, Feng X, Mugler JP, Meyer CH. SPRING-RIO TSE: 2D T2-Weighted Turbo Spin-Echo Brain Imaging using SPiral RINGs with Retraced In/Out Trajectories. Magn Reson Med. 2022;88:601-616.
[9] Alsop DC. Phase insensitive preparation of single‐shot RARE: application to diffusion imaging in humans. Magn Reson Med. 1997;38:527–533.
[10] Gao Y, Han F, Zhou Z, et al. Multishot diffusion‐prepared magnitude‐stabilized balanced steady‐state free precession sequence for distortion‐free diffusion imaging. Magn Reson Med. 2019;81:2374–2384.
[11] Van AT, Cervantes B, Kooijman H, Karampinos DC. Analysis of phase error effects in multishot diffusion-prepared turbo spin echo imaging. Quant Imaging Med Surg 2017;7(2):238-250.
[12] Fessler JA. Michigan image reconstruction toolbox (MIRT). https://web.eecs.umich.edu/~fessler/code/index.html. Accessed September 15, 2018.
[13] Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT–an eigenvalue approach to auto-calibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71:990–1001.
Figures
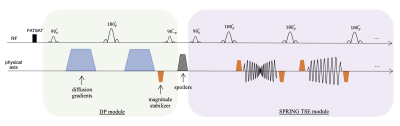
Figure 1. Sequence diagram of single-shot diffusion-prepared (DP) spiral-ring (SPRING) TSE with gradient stabilizers (blue trapezoids: diffusion gradients, orange trapezoids: magnitude stabilizers, striped trapezoids: spoiler gradients).

Table 1. Sequence parameters for SS-DP-SPRING TSE and SS-DW-EPI acquisitions.
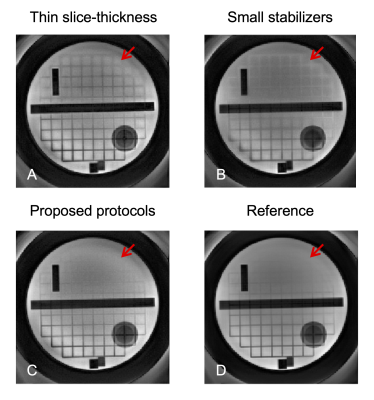
Figure 2. Phantom images showing the performance of a larger magnitude stabilizer (~4π dephasing) and a thicker slice-thickness (1.5X larger) used in the DP module (C) for cross-talk artifact reduction (red arrows), compared to 1X slice-thickness (A) and a 2π dephasing stabilizer (B), respectively. Note that the diffusion gradient was off, and data was fully sampled for all images.
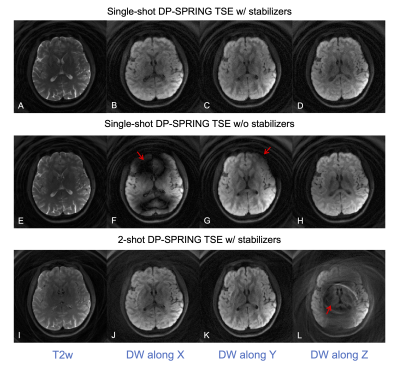
Figure 3. In vivo brain images showing the stability of image quality from the proposed single-shot DP-SRPING TSE sequence with magnitude stabilizers (A-D) over single-shot DP-SRPING TSE without stabilizers (E-H) and a 2-shot DP-SRPING TSE sequence with stabilizers (I-L). Note that all images were reconstructed via NUFFT, and images E-H have theoretical 2X SNR higher than other images. (NSA = 1).
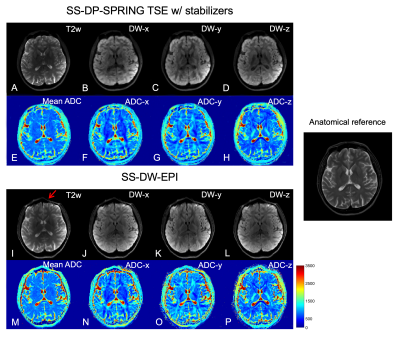
Figure 4. Example of single-shot in vivo results showing images at b-values of 0 (A, I) and 750 s/mm2 (B-D, J-L), ADC maps for three main directions (F-H, N-P), and mean ADC maps (E, M), acquired from SS-DP-SPRING TSE with magnitude stabilizers (A-H) and SS-DW-EPI (I-P). The conventional T2w image on the right is considered the anatomical reference. Red arrow points to distortion and artifacts in SS-DW-EPI. (NSA = 4 for SS-DP-SPRING TSE, 2 for SS-DW-EPI.)
DOI: https://doi.org/10.58530/2023/2018