2011
Respiratory binning with PilotTone Navigator For Motion Compensated Liver DW-MRI1HARVARD MEDICAL SCHOOL, BOSTON, MA, United States, 2RADIOLOGY, BOSTON CHILDREN'S HOSPITAL, HARVARD MEDICAL SCHOOL, BOSTON, MA, United States
Synopsis
Keywords: Motion Correction, Body
Respiratory motion substantially affects the accuracy of quantitative DWI-MR techniques in the upper abdomen. A conventional approach for motion correction in abdominal MRI uses a respiratory belt or other navigators for prospective triggering. This study explores use of a new approach - PilotTone (PT) navigator for binning.Introduction
Respiratory motion substantially affects the accuracy of quantitative DWI-MR techniques in the upper abdomen. A conventional approach for motion correction in abdominal MRI uses a respiratory belt or other navigators for prospective triggering. In the triggering method, data is acquired only in a small portion of the respiratory cycle and therefore this method prolongs scan time, which is undesirable in clinical practice. Recent retrospective methods used a respiratory belt for binning the signal into relatively stationary respiratory phases. However, the respiratory belt has limitations to provide an accurate motion signal for navigation and binning1. This study explores use of a new approach - PilotTone (PT) navigator for binning. PT is a reference signal generated by a small wireless RF transmitter, and has been used to track physiological motion2-3 PT signals are recorded to multichannel raw k-space data. To select the best PT channel for navigation and binning to optimize motion correction performance in the target region, we used a reference motion estimation method for guidance. Our reference method was based on motion tracking of sequentially acquired slices with a 3D slice-to-volume registration (SVR) method4-5.Methods
Data was acquired with a DW-MRI sequence on 5 volunteers using a 3T scanner (MAGNETOM Prisma, Siemens) with a free-breathing single-shot EPI sequence:TR/TE=5200/78ms;FOV=380x310mm;inplane resolution=1.5x1.5mm2;slice_thickness=4mm;BW=2442Hz/px;b-values=50,400,800 s/mm2 with 2,3,5 averages;6 directions. A PT transmitter box was placed on the patient table next to the hip of the volunteer. PT navigator signals were extracted from the raw data for each channel (PTcha) and it represents generic motion (no distinction is made with respect to translation or rotation in either of the three planes). We select the best PT channel for navigation and binning to optimise motion correction performance in the target region using a reference motion estimation method for guidance. Our method was based on a sequentially guided slice-to-volume registration with Kalman filtering to estimate 6 rigid body motion from the retrospectively analysed data4-5. Normalized cross correlation is computed between the two largest rigid motion components from SVR (translation along z and y axis) and PT signal in each channel. Channel with highest correlation is chosen as optimal PT signal for binning. Uniform binning was performed using this signal. Slices that shared the same bin were averaged together. Reconstructed volumes at each bin were registered to a reference bin via non-rigid registration6. The registered volumes from each bin were averaged to generate final motion corrected volumes at each b-value. The image quality of motion corrected volumes with the PT method were compared to volumes corrected with the reference SVR method, and to original (uncorrected) volumes. ADC parameters are estimated from the motion corrected data and compared to non motion corrected data, as well as the motion corrected data with PilotTone.Results
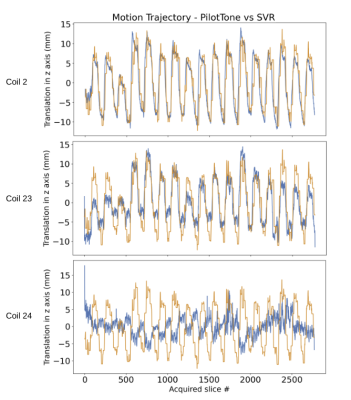
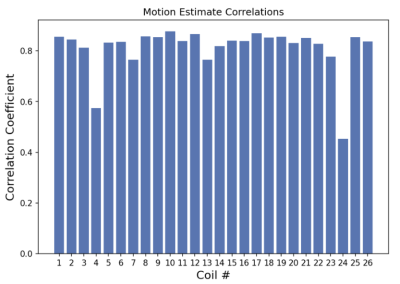
Figure 1: Temporal evolution of the RF signal recorded by PilotTone is shown for 3 coils from the same scan. Each coil is sensitive to specific direction of motion and region, which depends on its orientation and proximity to organs. SVR estimates of through slice motion trajectory, which is in the dorsal-ventral direction in axially acquired data, and is the largest component of motion, is overlaid on top of each PT coil in orange color lines. Cross correlation is estimated between SVR and PT coils to detect a coil that is most similar to through slice motion. Correlations shows are 0.84, 0.77 and 0.45.Figure 2: Cross correlation scores between each PT coil and SVR dorsal-ventral motion estimates for all coils. A coil with the highest correlation score is chosen for binned data reconstruction, which should yield the most coherence between slices that belong to the same respiratory cycle for through-slice motion.
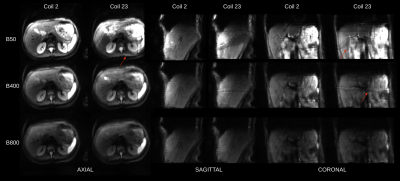
Figure 3: Reconstructions of b=50,400,800 b-value images that were guided by two selected PilotTone coil signal trajectories. Each reconstruction includes data sorting into respiratory phases according to the preselected coil, geometric averaging and non-rigid registrations between final respiratory cycle data bins. The final result are three images - b50,b400,b800. Coil 2 and Coil 23 correspond to coils in Figure 1. Reconstructed images are of significantly higher quality when a coil with higher cross correlation score is picked.
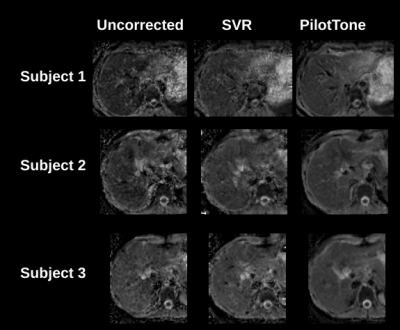
Figure 4: ADC maps estimated from three methods: a) free-breathing (uncorrected) images; b) motion corrected images with SVR algorithm only; c) motion corrected images reconstructed with PilotTone based strategy. Data is shown for 3 different subjects. PilotTone reconstructed ADC maps show superior quality in structure, which suggests that a large bulk of motion related artifacts were completely resolved.
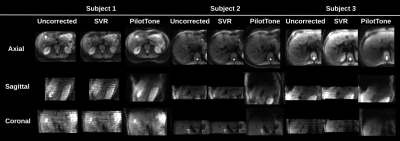
Figure 5: An example of b=400s/mm2 b-value image in three orthogonal planes for: a) free-breathing (uncorrected) b) motion corrected with SVR; c) motion corrected with proposed PilotTone strategy. Uncorrected and SVR corrected data is highly disturbed in the sagittal and coronal plane with evident stripy patterns for the case with severe motion in Subject 1. The motion effect is lesser in Subjects 2 and 3. PilotTone based method yields superior quality of reconstructed image in all cases.
Conclusions and Discussion
We evaluated the performance of our PT guided motion correction technique and shown improvement of the image quality before and after motion compensation.Acknowledgements
This work was supported in part by NIH grants R01 EB019483, R01 NS121657, R01 DK125561, R21 DK123569, R21 EB02962, S10OD025111 and a pilot grant (PP-1905-34002) from the National Multiple Sclerosis Society.References
1. Ehman, R.L., McNamara, M.T., Pallack, M., Hricak, H. and Higgins, C.B., 1984. Magnetic resonance imaging with respiratory gating: techniques and advantages. American journal of Roentgenology, 143(6), pp.1175-1182
2. Ludwig, Juliane, et al. "Pilot tone–based motion correction for prospective respiratory compensated cardiac cine MRI." Magnetic Resonance in Medicine 85.5 (2021): 2403-2416.
3. Solomon, Eddy, et al. "Free-breathing radial imaging using a pilot-tone radiofrequency transmitter for detection of respiratory motion." Magnetic resonance in medicine 85.5 (2021): 2672-2685.J. Coll-Font, et al. Retrospective Distortion and Motion Correction for Free‐Breathing DW‐MRI of the Kidneys Using Dual‐Echo EPI and Slice-to-Volume Registration. Journal of Magnetic Resonance Imaging, 2020
4. S. Kurugol, et al. Motion-Robust Spatially Constrained Parameter Estimation in Renal Diffusion-Weighted MRI by 3D Motion Tracking and Correction of Sequential Slices, MICCAI RAMBO workshop, Lecture Notes in Computer Science, vol 10555. Springer, 2017.
5. O. Commowick, et al. Automated diffeomorphic registration of anatomical structures with rigid parts: application to dynamic cervical MRI. MICCAI, pp.163-70, 2012.
Figures