2010
Reduction of motion artifacts in diffusion weighted brain images using first order motion-compensated diffusion gradients1Cardiovascular Innovation Research Center, Cleveland Clinic, Cleveland, OH, United States, 2Imaging Institute, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Keywords: Motion Correction, Diffusion/other diffusion imaging techniques, Diffusion Tensor Imaging, Motion Correction, Neuro
Patient motion during acquisition of diffusion weighted (DW) images causes significant signal dropout. To perform motion correction, DW images of the brain were acquired during gross head motion using a first order motion-compensated diffusion gradient scheme and compared to a traditional DW-EPI sequence. Of the images acquired with the motion-compensated sequence, only 1.5% contained significant signal dropout compared to 36% of the images acquired with the traditional sequence. Overall, the motion-compensated sequence reduced signal dropout by 96% compared to the traditional sequence without increasing scan time or introducing new post-processing protocols.Introduction
Diffusion tensor imaging (DTI) is a valuable tool for studying a variety of neurological conditions, including multiple sclerosis, Parkinson’s disease, Huntington’s disease, and stroke1. Patient populations such as these are highly susceptible to involuntary head motion during scanning, making it challenging to acquire quality DTI data due to the sensitivity of diffusion weighted imaging sequences to motion-related signal dropout2-4. Many prospective and retrospective techniques have been proposed to reduce motion artifacts in DTI data, but these techniques commonly require prohibitive increases in scan time and post-processing computation5-11. Currently, the most effective means of reducing motion artifacts in DTI data involve restricting head motion, discarding corrupted data, and averaging over multiple replicate volumes1.Previous work has demonstrated that it is possible to perform DTI in the living heart — an organ that exhibits motion to the extreme as compared to the head12-14. The strategy uses motion-compensated diffusion weighting (DW) gradients and incurs no penalty in scan time. However, this approach has not, to our knowledge, been applied to the brain. Here, we investigate the use of this strategy in the context of gross head motion.
Methods
A second order motion-compensated (M2) diffusion gradient scheme has been successfully implemented by Nguyen et al. to perform free-breathing diffusion tensor cardiac MRI12-14. Based on the success of the M2 cardiac DTI sequence in capturing high quality diffusion data despite respiratory motion and the motion of the heart, this work implements and tests a diffusion weighted sequence with a first order motion-compensated (M1) diffusion gradient scheme for robustness to gross head motion.Under an IRB-approved protocol, a healthy volunteer was scanned using a diffusion weighted (DW) single shot echo planar imaging (EPI) sequence with first order motion-compensated gradients. For comparison, the volunteer was also scanned using a traditional DW-EPI sequence. Both scans were performed with a 3T Siemens Prisma (Siemens Healthineers, Erlangen) (TR/TE = 14700/77 ms, field of view (FOV) = 220 × 220 mm2, 110 × 110 matrix, 2 mm slice thickness, 60 slices, b = 1000 s/mm2 with DW gradients applied only in the x-direction, TA = 6 min). Eighteen replicate image volumes were acquired for both experiments. The volunteer was instructed to lie still during the first 9 image volumes and to tilt his head side-to-side within the coronal plane for the last 9 image volumes for each sequence.
Results
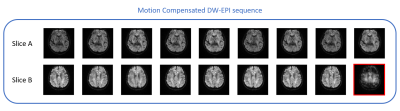
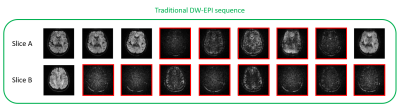
The diffusion weighted images acquired during head motion for two representative slices are shown in Figures 1 and 2. Results obtained using the motion compensated sequence are shown in Figure 1 and results obtained using the traditional sequence are shown in Figure 2. In both figures, images corrupted by motion-related signal dropout are highlighted in red.As is evident in Figure 1, none of the 9 images acquired for the first representative slice (Slice A) using the motion-compensated sequence show significant signal dropout and only 1 of the 9 images acquired during head motion was corrupted by signal dropout in the second representative slice (Slice B). In contrast, Figure 2 shows that for the first representative slice (Slice A), 5 out of the 9 images acquired during active head motion using the traditional sequence are significantly corrupted by signal dropout. In Slice B, 8 of 9 images collected during head motion were corrupted by signal dropout.
The overall results of this study are summarized in Table 1. Only 1.5% of the images collected during head motion using the motion-compensated sequence were corrupted by significant signal dropout. In contrast, 36% of the images collected during head motion using the traditional sequence were corrupted.
Discussion
Implementation of the DW-EPI sequence with a motion compensated gradient scheme compared to the traditional sequence reduced the number of scans that were corrupted by motion-induced signal dropout by 96%. This substantially improved robustness to motion artifacts was achieved without increasing scan duration or introducing complicated post-processing computation.In this proof-of-principle study, diffusion gradients were only applied in the x-direction. To calculate the full set of diffusion tensor parameters, additional diffusion weighted data must be collected. Based on the findings of this work, the implementation of motion-compensated gradients in orthogonal directions will allow for the collection of the high-quality diffusion weighted image data needed to perform DTI imaging of the head in the presence of head motion.
Conclusion
In this study, we successfully acquired the quality DW data required to calculate DT parameters in the presence of head motion by implementing a first-order motion compensated gradient scheme. Unlike many approaches used to mitigate motion-related signal dropout in diffusion weighted image data that discard corrupted data and add on additional scans to get the necessary complete data set, this technique is able to acquire quality data while the head is moving. Due to this capability, the motion-compensated DW-EPI approach will dramatically improve the outcome of diffusion-weighted imaging for patient populations with a high susceptibility to gross head motion. This approach also has the potential to address pulsatility artifacts, which affect even subjects who are otherwise able to lie still.Acknowledgements
No acknowledgement found.References
1. Soares, J., Marques, P., Alves, V., & Sousa, N. (2013). A hitchhiker's guide to diffusion tensor imaging. Frontiers in Neuroscience, 7.
2. Havsteen, I., Ohlhues, A., Madsen, K. H., Nybing, J. D., Christensen, H., & Christensen, A. (2017). Are Movement Artifacts in Magnetic Resonance Imaging a Real Problem?-A Narrative Review. Frontiers in neurology, 8, 232. https://doi.org/10.3389/fneur.2017.00232
3. Zaitsev, M., Maclaren, J. and Herbst, M. (2015), Motion artifacts in MRI: A complex problem with many partial solutions. J. Magn. Reson. Imaging, 42: 887-901. https://doi.org/10.1002/jmri.24850
4. Baum, G. L., Roalf, D. R., Cook, P. A., Ciric, R., Rosen, A., Xia, C., Elliott, M. A., Ruparel, K., Verma, R., Tunç, B., Gur, R. C., Gur, R. E., Bassett, D. S., & Satterthwaite, T. D. (2018). The impact of in-scanner head motion on structural connectivity derived from diffusion MRI. NeuroImage, 173, 275–286. https://doi.org/10.1016/j.neuroimage.2018.02.041
5. Taylor, P. A., Alhamud, A., van der Kouwe, A., Saleh, M. G., Laughton, B., & Meintjes, E. (2016). Assessing the performance of different DTI motion correction strategies in the presence of EPI distortion correction. Human brain mapping, 37(12), 4405–4424. https://doi.org/10.1002/hbm.23318
6. Stefan Skare, & Jesper L.R. Andersson (2001). On the effects of gating in diffusion imaging of the brain using single shot EPI. Magnetic Resonance Imaging, 19(8), 1125-1128.
7. Mohammadi, S., Hutton, C., Nagy, Z., Josephs, O., & Weiskopf, N. (2013). Retrospective correction of physiological noise in DTI using an extended tensor model and peripheral measurements. Magnetic resonance in medicine, 70(2), 358–369. https://doi.org/10.1002/mrm.24467
8. Benner, T., van der Kouwe, A. J., & Sorensen, A. G. (2011). Diffusion imaging with prospective motion correction and reacquisition. Magnetic resonance in medicine, 66(1), 154–167. https://doi.org/10.1002/mrm.22837
9. Kober, T., Gruetter, R., & Krueger, G. (2012). Prospective and retrospective motion correction in diffusion magnetic resonance imaging of the human brain. NeuroImage, 59(1), 389–398. https://doi.org/10.1016/j.neuroimage.2011.07.004
10. Alhamud, A., Tisdall, M. D., Hess, A. T., Hasan, K. M., Meintjes, E. M., & van der Kouwe, A. J. (2012). Volumetric navigators for real-time motion correction in diffusion tensor imaging. Magnetic resonance in medicine, 68(4), 1097–1108. https://doi.org/10.1002/mrm.23314 Alhamud, A., Taylor, P. A., Laughton, B., van der Kouwe, A. J., & Meintjes, E. M. (2015). Motion artifact reduction in pediatric diffusion tensor imaging using fast prospective correction. Journal of magnetic resonance imaging : JMRI, 41(5), 1353–1364. https://doi.org/10.1002/jmri.24678
11. Nguyen, C., Fan, Z., Sharif, B., He, Y., Dharmakumar, R., Berman, D.S. and Li, D. (2014), In vivo three-dimensional high resolution cardiac diffusion-weighted MRI: A motion compensated diffusion-prepared balanced steady-state free precession approach. Magn. Reson. Med., 72: 1257-1267. https://doi.org/10.1002/mrm.25038
12. Nguyen, C., Fan, Z., Xie, Y., Pang, J., Speier, P., Bi, X., Kobashigawa, J., & Li, D. (2016). In vivo diffusion-tensor MRI of the human heart on a 3 tesla clinical scanner: An optimized second order (M2) motion compensated diffusion-preparation approach. Magnetic resonance in medicine, 76(5), 1354–1363. https://doi.org/10.1002/mrm.26380
13. Nguyen, C., Christodoulou, A., Coll-Font, J., Ma, S., Xie, Y., Reese, T., Mekkaoui, C., Lewis, G., Bi, X., Sosnovik, D., & Li, D. (2021). Free-breathing diffusion tensor MRI of the whole left ventricle using second-order motion compensation and multitasking respiratory motion correction. Magnetic Resonance in Medicine, 85(5), 2634-2648.
Figures