2003
Detection and remediation of ghost artifacts in low b-value diffusion MRI with ghost re-synthetization and 4-way phase-encoded data1Catholic University of America, Washington, DC, United States, 2QMI, NIBIB/National Institutes of Health, Bethesda, MD, United States
Synopsis
Keywords: Artifacts, Artifacts, diffusion mri
We propose a novel post-processing method for ghost artifact correction in low-b diffusion weighted images (DWIs) using ghost synthetization and four different phase-encoding direction (PED) images. The results from both simulations and real data show that our method can robustly detect and correct ghost artifacts on b=0 s/mm2 images for artifact-free image. The proposed method can be used further to generate ground truth training images for a machine-leaning method to remedy ghost artifacts in single PED acquisition.Introduction
Diffusion-weighted images (DWIs) are typically acquired using echo planar imaging (EPI), which is known to suffer from ghost artifacts. Most ghost artifact correction methods1,2 require additional information such as navigator signals, reference scans, or raw k-space signals. Although machine learning algorithms3 can overcome such limitations, large training datasets are often needed. Ghost artifacts manifest themselves along the phase-encoding direction (PED), therefore for datasets acquired with different PEDs, such artifacts would be localized at different spatial positions4. With thoroughly preprocessed DWIs, the voxelwise variance among different PED images would ideally be a function of noise, except for voxels affected by ghosts, where it would be artificially higher. However, in low b-value dMRI, variance values might spatially vary due to factors such as CSF flow or imperfect distortion correction. In this work, we propose a robust post-processing-based technique for low b-value ghost artifact detection/remediation methodology that uses data acquired with four PEDs such as Anterior-Posterior (AP), Posterior-Anterior (PA), Right-Left (RL), and Left-Right (LR).Materials and method
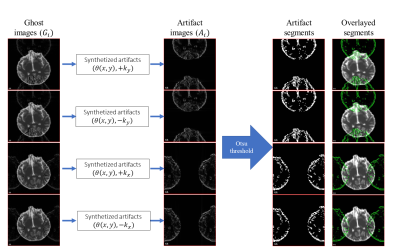
Method: The proposed method combines the analysis of variance (ANOVA) on population group means (i.e., $$$H_0: \mu_{LR} = \mu_{RL} = \mu_{AP} = \mu_{PA}$$$) with a synthetic ghost generation strategy. Rejection of the null hypothesis indicates the likelihood of a ghost artifact. Ghost synthetization step aims to constrain the application of ANOVA to only voxels that might contain ghosts to reduce the false positive detection rate in artifact-free but high variance regions such as the CSF. The synthetic ghost map is based on constant N/2 shifting and mapping characteristic of SENSE. Ghost artifacts are simulated by phase mismatch between odd and even lines in k-space. The proposed method that allows us to map potential ghost voxels in images, is described in Eq. (1)$$ S'(k_y, k_y)= \begin{cases}L_{odd}.\theta(x,y)\\L_{even}.(-(\theta(x,y))\end{cases} (1)$$
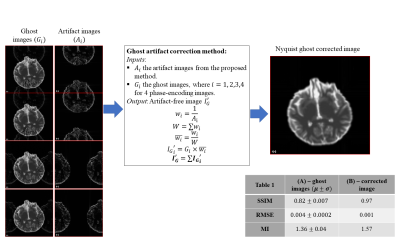
where, $$$\theta(x,y)$$$ is the phase-error modeled in the convention of Buonocore and Gao5. We denote $$$L_{odd}$$$ and $$$L_{even}$$$ as odd and even lines respectively on the phase-encoding axis of the image,$$$S'(k_x, k_y)$$$is the manipulated signal in the frequency domain. In the remainder of this abstract, “ghost images” refer to the actual images that might suffer from ghost artifacts, and “artifact images” refer to synthetic ghost maps. Figure 1 shows our proposed pipeline for Nyquist ghost artifact detection using the artifact images. Figure 2 shows the ghost artifact correction method on simulated data. For the simulated data, ANOVA is not applied for detection. Instead, Otsu thresholding6 is used to segment artifact voxels. For real data, the percent contribution of within-group variance to total variance is again thresholded to generate final voxel artifacts.
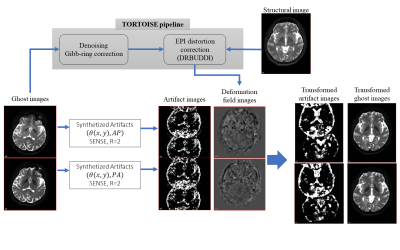
Dataset: Two datasets were used in this project. The first dataset is a simulated EPI dataset from FSL-POSSUM7 with known artificial ghost artifacts. The second one is the real dataset with 4-way PED dMRI data, as described in [4], which also contains a fat-suppressed T2W-TSE image. Five longitudinal scans of a single subject were used for testing. The proposed method was tested only on the b=0 images and not diffusion-weighted volumes. DWI pre-processing was performed with the TORTOISE pipeline8: The susceptibility distortion differences among PEDs were virtually eliminated, leaving virtually only the ghost artifacts present in the data. All DWIs were rigidly aligned to the T2W structural image of the subject’s first scan session. The resulting transformations are also applied to the native-space artifact images to determine the candidate voxels in the final space. Figure 3 illustrates the pipeline implementation.
Results
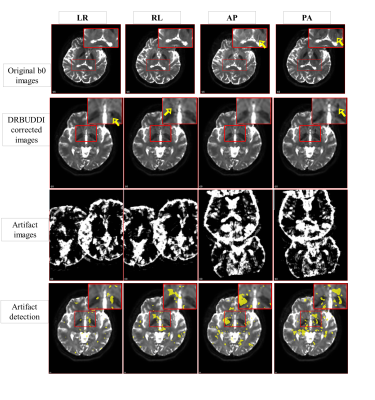
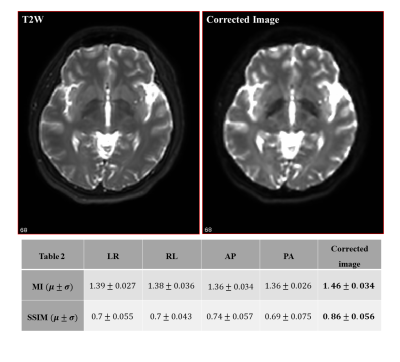
The root means square error (RMSE), structural similarity (SSIM), and mutual information (MI) metrics were used to evaluate our method. For the simulated dataset, the ghost-free image is considered as a ground truth. Table 1 in Figure 2 displays three evaluation metrics. It is clear that the method successfully eliminated most of the ghost artifacts. The computed SSIMs between the ghost-free image and the corrected image was significantly increased and reached 0.97. Figure 4 shows our ghost artifact detection on the real dataset. The images in the second row are processed and transformed images, that still suffer from ghosts. The yellow arrows here indicate observed artifactual regions. The third row displays the transformed synthetic ghost images, i.e. candidate ghost voxels, and the fourth row the final outcome of statistical ghost detection. The detected ghost voxels match very well with artifactual regions after visual inspection. For quantitative validation, the ghost corrected images were compared to the T2W image. Table 2 in Figure 5 shows the computed SSIM and MI values across 4 PED and 5 scans, both SSIM and MI were significantly improved (16% to 24%) after the ghost correction.Conclusion and discussion
We proposed and validated a robust and easy-to-implement method that can reliably correct for ghost artifacts on low b-value dMRI when 4-way PED data is available. The model can be further improved for high b-value shells by combining it with existing robust fitting techniques10,11 for artifact detection. As a future work, the proposed technique will be used to generate “ground-truth” ghost map data, which will be employed in a machine learning framework to detect the artifacts for single or dual phase-encoded images.Acknowledgements
The authors would like to thank Dr. Neville D. Gai for the technical instructions on the Nyquist simulation.References
1. Heid O, inventor; Siemens Healthcare GmbH, assignee. Method for the phase correction of nuclear magnetic resonance signals. US patents 6,043,651. Mar 28, 2000.
2. McKay, J. A., Moeller, S., Zhang, L., Auerbach, E. J., Nelson, M. T., Bolan, P. J. Nyquist ghost correction of breast diffusion weighted imaging using referenceless methods. Magnetic resonance in medicine. 81(4), 2624-2631, 2019.
3. Lee J, Han Y, Ryu JK, Park JY, Ye JC. k-Space deep learning for reference-free EPI ghost correction. Magn Reson Med. 2019 Dec;82(6):2299-2313. doi: 10.1002/mrm.27896. Epub 2019 Jul 18. PMID: 31321809.
4. Irfanoglu MO, Sadeghi N, Sarlls J, Pierpaoli, C, Improved Reproducibility of Diffusion MRI of the Human Brain with a Four-way Blip-up and Down Phase-Encoding Acquisition Approach, Magnetic Resonance in Medicine, 2020.
5. Buonocore MH, Gao L. Ghost artifact reduction for echo planar imaging using image phase correction. Magn Reson Med. 1997 Jul;38(1):89-100. doi: 10.1002/mrm.1910380114. PMID: 9211384.
6. N. Otsu, "A Threshold Selection Method from Gray-Level Histograms," in IEEE Transactions on Systems, Man, and Cybernetics, vol. 9, no. 1, pp. 62-66, Jan. 1979, doi: 10.1109/TSMC.1979.4310076.
7. Mark S. Graham, Ivana Drobnjak and Hui Zhang. Realistic simulation of artefacts in diffusion MRI for validating post-processing correction techniques, NeuroImage 125, 1079-1094, 2016.
8. Pierpaoli C, Walker L, Irfanoglu MO, Barnett AS, Chang LC, Koay CG, et al. TORTOISE: An integrated software package for processing of diffusion MRI data. In: Proceedings of International Society of Magnetic Resonance in Medicine; 2010. p. 1597.
9. Irfanoglu MO, Modi P, Nayak A, Hutchinson EB, Sarlls J, Pierpaoli C. DR-BUDDI: (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. Neuroimage 2015;106:284–289
10. Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med. 2005;53(5):1088-1095. doi:10.1002/mrm.20426
11. Chang LC, Walker L, Pierpaoli C. Informed RESTORE: A method for robust estimation of diffusion tensor from low redundancy datasets in the presence of physiological noise artifacts. Magn Reson Med. 2012;68(5):1654-1663. doi:10.1002/mrm.24173
Figures