1993
Out-of-phase Ventiilation Observed With Dynamic Hyperpolarized Xenon-129 Mri In A Pig Thoracic Insu!ciency Syndrome Model
Hooman Hamedani1, Faraz Amzajerdian1, Stephen Kadlecek1, Kai Ruppert1, Mostafa Ismail1, Luis Loza1, Ian Duncan1, Madeline Boyes2, Klaus Hopster2, Rachel Hilliard2, Thomas Schaer2, Benjamin Sinder 3, Brian Snyder4, Axel Moore5, Dawn Elliott5, Kyle Meadows5, and Rahim Rizi1
1University of Pennsylvania, Philadelphia, PA, United States, 2University of Pennsylvania School of Veterinary Medicine, Kennett Square, PA, United States, 3Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 4Boston Children’s Hospital, Boston, MA, United States, 5University of Delaware, Newark, DE, United States
1University of Pennsylvania, Philadelphia, PA, United States, 2University of Pennsylvania School of Veterinary Medicine, Kennett Square, PA, United States, 3Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 4Boston Children’s Hospital, Boston, MA, United States, 5University of Delaware, Newark, DE, United States
Synopsis
Keywords: Body, Skeletal
In a pilot study of thoracic insufficiency syndrome in a pig model, we demonstrated dynamic hyperpolarized xenon-129 (HXe) MRI’s ability to noninvasively measure respiratory dysfunction in terms of gas distribution and mixing in the lungs, and specifically showed ‘out of phase’ ventilation in constricted regions of the lung. This marker of ventilation—i.e., the temporal characteristic of signal build-up during inhalation and its wash-out during exhalation—is inaccessible without our dynamic imaging technique. This technique can be implemented clinically to enhance our understanding of TIS pathophysiology and evaluate treatment strategies aimed at fostering age appropriate alveolarization.Introduction
Thoracic insufficiency syndrome (TIS) is caused by constrictive thoracic deformities that impede pulmonary function; a restrictive condition that prevents uniform lung inflation due to mechanically restricted chest wall and diaphragmatic motion, as well as impaired respiration resulting from compromised lung development—e.g., retarded alveolarization and neovascularization, increased septal wall thickness—TIS can cause lifelong functional impairment and significantly increased mortality due to respiratory and cardiovascular failure [1-3]. While the pathophysiology of TIS is not yet comprehensively defined, it is likely that abnormal tissue stretch, oxygenation and perfusion prevent normal lung maturation in affected children. CT offers several imaging tools to quantify aspects of pulmonary structure that are impacted by the anatomical abnormalities associated with TIS. We hypothesize that assessments of pulmonary function using dynamic HXe MRI permit the quantification of normal lung maturation, deviations or retardations in lung development caused by TIS, and functional improvements secondary to surgical intervention in an animal model. In this pilot study, we demonstrated hyperpolarized xenon-129 (HXe) MRI’s ability to noninvasively measure respiratory dysfunction in-vivo using an established pig model of TIS.Material and Methods
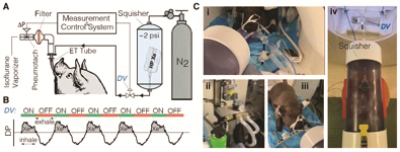
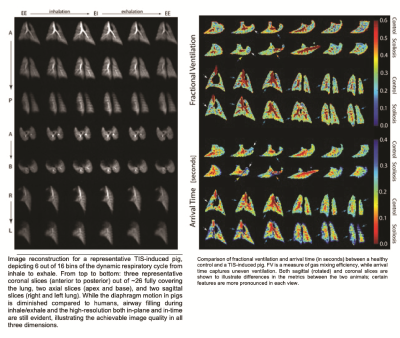
Two Yucatan miniature pigs were imaged, one healthy control and one TIS model. In the latter, the right hemithorax was constricted by tethering ribs 3-9 at 6-wk-old. At adulthood (5 months), imaging of the lungs (1.5T Siemens clinical MRI with custom HXe birdcage coil) was performed on intubated pigs breathing 30%O2+50ml HXe administered for ~50 breaths at 6mL/kg tidal volume (TV), as shown in Figure 1A. Animals were anesthetized, intubated, and connected to an isoflurane vaporizer through which a normoxic mixture of room air and isoflurane freely flowed, a bi-directional Pneumotach (AccutachTM, B & B Medical Technologies, USA), and a bacterial/viral filter. Imaging gas was dispensed into Tedlar® bags (Jensen Inert, USA) and placed under ~2psi pressure inside a custom-built plastic container (squisher) directly connected to the endotracheal (ET) tube by a ⅛” tube blocked by a pneumatic diaphragm valve that is normally closed (Parker Hannifin, USA). Custom-made software collected inhale/exhale breathing curves from the pneumotach and actuated the diaphragm valve on the imaging gas line during inhalation. Opening the pneumatic valve during inhalation at each breath delivered a small dose of HXe (~50 ml) through the tube connected to the ET tube. Figure 1B shows inhale/exhale breathing curves and the valve actuation scheme. Prior to each study, the pressure in the container was slightly adjusted to calibrate the amount of imaging gas to be delivered. In the pig studies reported here, a total of 2 L of HXe was delivered during a complete 50-breath cycle (40 ml of HXe per breath). Fig. C3C shows the gas delivery set-up, diaphragm valve, and squisher in the MRI room. Due to the small volumes of HXe administered per breath, we can image animals during spontaneous breathing and use signal averaging to produce lung maps of ventilation and gas exchange that are reflective of the animal’s actual breathing pattern. We collected 3D data sets of lung gas phase signal using an isotropic 3D spiral sequence: 640 unique interleaves, 512 samples per spiral, reconstructed on an 80×80×80 Cartesian grid, TR/TE = 7.63/0.62 ms, FA = 4° for a typical resolution of 4.4×4.4×4.4 mm3. Dynamic Gas Ventilation in the form of regional functional residual capacityFRC and TV were then evaluated, and fractional ventilation (FV) and arrival time (t) were quantified to show how well fresh gas mixes with residual gas in the lungs (FV) and how fast fresh gas reaches the parenchyma relative to large airways (t), thus quantifying uneven ventilation.Results and Discussion
Figure 2 shows reconstructed dynamic images in a TIS-induced pig, while Figure 3 compares maps of FV and t with those of a control animal. FV is slightly higher in the TIS model, probably due to the overall lung compression, which reduces the regional functional residual capacity (FV = TV/(TV+FRC)). Aside from the slightly lower global FV in the TIS model, the gas mixing efficiency (measured by FV) in both animals is more or less homogenous throughout the lungs. On the other hand, there are lung regions in the TIS pig with normal gas mixing efficiency but substantially higher and more heterogenous arrival times, which manifests as uneven ventilation (blue arrows). The white arrows indicate regions in the lung where both FV and arrival time are relatively low compared to the healthy animal and/or healthier regions of the parenchyma. In a large segment of the lung (orange arrow), ventilation is out of phase while FV is normal. Another peculiar feature in the TIS pig is the shorter gas arrival time at the fissure between the upper and lower lobes (yellow arrow).Conclusion
HXe MRI demonstrated non-uniform lung ventilation in a TIS model which would be inaccessible without the dynamic imaging approach used. This technique can be implemented clinically to enhance our understanding of TIS pathophysiology and evaluate treatment strategies aimed at fostering age appropriate alveolarization.Acknowledgements
No acknowledgement found.References
1 Campbell, R. M., Jr. & Smith, M. D. Thoracic insufficiency syndrome and exotic scoliosis. J Bone Joint Surg Am 89 Suppl 1, 108-122 (2007). https://doi.org:10.2106/jbjs.F.002702
2 Campbell, R. M., Jr. et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am 85, 399-408 (2003). https://doi.org:10.2106/00004623-200303000-000013
3 Mayer, O., Campbell, R., Cahill, P. & Redding, G. Thoracic Insufficiency Syndrome. Curr Probl Pediatr Adolesc Health Care 46, 72-97 (2016). https://doi.org:10.1016/j.cppeds.2015.11.001
DOI: https://doi.org/10.58530/2023/1993