1985
Comparison of 3D Ultra-short Echo Time (UTE) Phase-Resolved Functional Lung (PREFUL) with Hyperpolarized 129Xe MRI in Pediatric Cystic Fibrosis1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Division of Respiratory Medicine, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
Keywords: Data Processing, Lung
2D phase-resolved functional lung (PREFUL) MRI has been shown to correlate with hyperpolarized Xenon MRI (Xe-MRI) in pediatric Cystic Fibrosis (CF) lung disease but ventilation defects may be missed using the 2D approach. In this study we develop a 3D ultra-short echo time (UTE) PREFUL MRI method and compare to 2D PREFUL MRI and Xe-MRI in CF. 3D UTE PREFUL MRI showed a lower bias between Xe-MRI as compared to 2D PREFUL and was able to detect ventilation defects missed by the 2D approach, suggesting that the 3D approach may be a more comprehensive measure of ventilation in pediatric CF.Introduction
Hyperpolarized 129Xe MRI (Xe-MRI) provides regional pulmonary ventilation defect percent (VDP) measures and has been shown to detect early Cystic Fibrosis (CF) lung disease1. However, Xe-MRI requires a breath-hold, which can be challenging in young children. Phase-resolved functional lung (PREFUL)2 is a free-breathing MRI technique that does not require lengthy breath-holds of hyperpolarized gas. 2D PREFUL has been shown to correlate with Xe-MRI3 and is a responsive measure of pulmonary exacerbation treatment in pediatric CF4. However, out-of-slice ventilation defects may be missed using the 2D approach, motivating the need for 3D PREFUL approaches. While a pseudo-3D stack-of-stars acquisition has been shown to generate repeatable PREFUL MRI ventilation maps in COPD and healthy adults5, the inclusion of ultra-short echo time (UTE) may further improve signal-to-noise ratio in the lung tissue, where T2* is short. PREFUL with a 2D UTE sequence has shown to be feasible in healthy adults6 but has not been explored in pediatric CF patients. The purpose of this work was to obtain ventilation maps and VDP values using 3D UTE PREFUL in pediatric CF lung disease and compare them to similar measures obtained with multi-slice Xe-MRI and pulmonary function tests.Methods
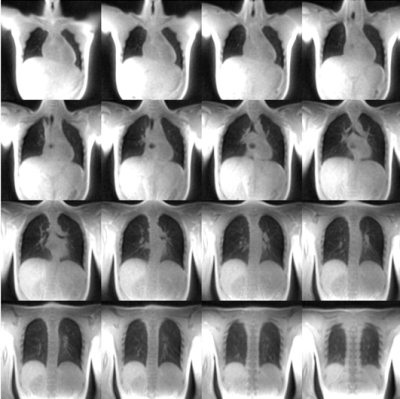
3D UTE PREFUL was performed in 8 CF and 4 healthy participants aged 15±2 years old using REB-approved protocols. 5 CF patients had 2 visits each, pre- and post-CFTR-modulator treatment. Subjects performed N2 multiple breath washout to obtain lung clearance index (LCI) and spirometry to obtain forced expiratory volume in one second (FEV1 % pred.). Multislice Xe-MRI and 2D free-breathing MRI were also performed, as previously described3. The 3D UTE sequence used a 20µs non-selective hard pulse and a center-out 3D golden-means radial trajectory7 with ramped sampling. The acquisition was performed with the following scan parameters: TR 1.92ms, TE 0.05ms, FOV 500x500x500 mm2, BW/pixel 830Hz/pixel, flip angle 3º. 280,000 radial spokes were acquired for a total acquisition time of 8 minutes and 58 seconds. For each participant, the DC signal of a single coil element closest to the diaphragm was used to sort the spokes into 30 respiratory phases. Each respiratory phase was reconstructed with ~21000 spokes. Using MATLAB (MathWorks, Natick, MA), each respiratory phase was reconstructed to a resolution of 1.95x1.95x1.95mm3 by applying iterative SENSE with 3D total variation and 3D Symlet 4 wavelet regularization in the spatial dimensions. Each image was bias-corrected using an N4 Bias Field Correction (using 3D Slicer8) and registered to the expiration phase using a demons-based non-rigid registration9. Representative lung images at expiration for a pediatric healthy participant are shown in Figure 1. The thoracic cavity was segmented using a 3D seeded region-growing algorithm. Following the PREFUL algorithm2,10, 15 equidistant phases were interpolated using non-parametric regression. A 3D image-guided filter was applied to each phase and the regional ventilation (RVent) of each voxel within the thoracic cavity mask was determined. As a comparator, single-slice 2D RVent maps were obtained from 2D free breathing 1H MRI as described by Voskrebenzev et al.2. Xenon ventilation maps were determined from Xe-MRI6. VDP was calculated from 2D/3D RVent maps using K-means clustering11 and from xenon ventilation maps using a threshold of <60% of the mean whole-lung signal12. Bland-Altman analysis was used to assess bias between VDP3D-RVent, VDP2D-Rvent, and VDPXe. VDP3D-RVent, VDP2D-Rvent, VDPXe, FEV1, and LCI were correlated using linear regression.Results
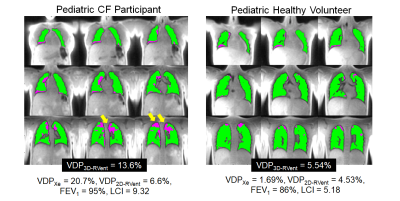
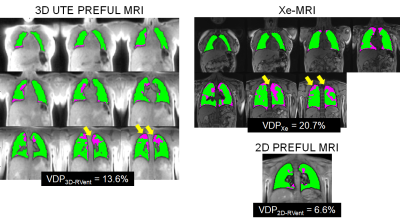
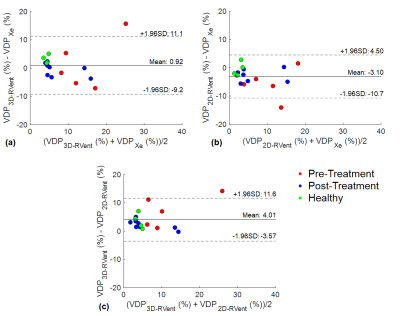
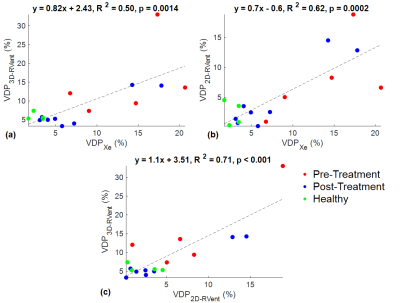
Figure 2 shows the VDP3D-Rvent maps for a representative CF patient pre-CFTR treatment and healthy pediatric control. Figure 3 shows the MRI-derived defect maps for a representative CF patient in which ventilation defects were missed by 2D PREFUL (arrows). Bland-Altman analysis between VDP3D-RVent, VDP2D-Rvent, VDPXe in pediatric CF patients (pre-and post-CFTR modulator treatment) and healthy controls are shown in Figure 4. A mean bias of 0.92 [-9.2,11.1] was found between VDP3D-RVent and VDPXe, and -3.10 [-10.7,4.5] between VDP2D-RVent and VDPXe. Correlation plots between Xe-MRI and PREFUL are shown in Figure 5. VDP3D-RVent, VDP2D-RVent, and VDPXe all significantly correlated to LCI (all p<0.01) but not with FEV1 (all p>0.05).Discussion
3D UTE PREFUL was successfully performed in all participants and detected ventilation defects in pediatric CF patients not captured by 2D PREFUL. VDP3D-RVent showed a lower absolute bias between VDPXe as compared to VDP2D-RVent, reflective of the ability of 3D UTE PREFUL to detect more ventilation defect burden throughout the entire lung. However, the strength at which VDP3D-Rvent correlated with VDPXe was lower than VDP2D-Rvent. The lack of an increase in correlation strength may be because Xe-MRI measures the amount of intrapulmonary gas mixing while PREFUL MRI measures the overall change of lung tissue density during tidal breathing, and thus may lead to differences in the relative defects detected by the two approaches throughout the entire lung. Improvements to the 3D PREFUL MRI approach include increased under sampling of k-space to reduce scan time, similar to the work of Klimes et al10, reducing acquisitions to ~4mins is of particular importance in children who are prone to movement.Conclusion
Whole lung ventilation assessment with 3D UTE PREFUL MRI provides more comprehensive evaluation of lung function compared to single-slice 2D PREFUL and agrees well Xe-MRI in pediatric CF.Acknowledgements
We would like to thank the following sources of funding: The Hospital for Sick Children, Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (RGPIN 217015-2013), the Cystic Fibrosis Foundation (CFF), Canadian Institutes of Health Research (CIHR) operating and project grants (MOP 123431, PJT 153099). Samal Munidasa would like to thank Restracomp and NSERC for their support.References
1. Santyr G, Kanhere K, Morgado F, et al. Hyperpolarized Gas Magnetic Resonance Imaging of Pediatric Cystic Fibrosis Lung Disease. Acad Radiol. 2019;26(3):344-354.
2. Voskrebenzev A, Gutberlet M, Klimes F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med. 2018;79(4):2306-2314.
3. Couch MJ, Munidasa S, Rayment J, et al. Comparison of Functional Free-Breathing Pulmonary 1H and Hyperpolarized 129Xe Magnetic Resonance Imaging in Pediatric Cystic Fibrosis. Acad Radiol. 2021;28(8): e209-e218.
4. Munidasa S, Couch MJ, Rayment J, et al. Free-breathing MRI for Monitoring Ventilation Changes following Antibiotic Treatment of Pulmonary Exacerbations in Pediatric Cystic Fibrosis. Eur. Respir. J. 2021;57(4):2003104
5. Klimes, F., Voskrebenzev, A., Gutberlet, et al. Repeatability of dynamic 3D phase-resolved functional lung (PREFUL) ventilation MR Imaging in patients with chronic obstructive pulmonary disease and healthy volunteers. J Magn Reson Imaging. 2021 Aug;54(2):618-629. doi: 10.1002/jmri.27543. Epub 2021 Feb 9. PMID: 33565215.
6. Behrendt L, Gutberlet M, Voskrebenzev A, et al. Feasibility of phase resolved functional lung imaging (PREFUL) with ultrashort echo time sequence (PUTE). Proc. Intl. Soc. Mag. Reson. Med. 30 (2022):1402 7. Feng L. Golden-Angle Radial MRI: Basics, Advances, and Applications. J. Magn. Reson. Imaging 2022;56:45–62.
8. Fedorov A., Beichel R., Kalpathy-Cramer J., et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012 Nov;30(9):1323-41. PMID: 22770690. PMCID: PMC3466397.
9. Daniel Forsberg (2022). fordanic/image-registration (https://github.com/fordanic/image-registration), GitHub. Retrieved October 26, 2022.
10. Klimes F, Voskrebenzev A, Gutberlet M, Kern AL, Behrendt L, Grimm R, Suhling H, Crisosto CG, Kaireit TF, Pöhler GH, Glandorf J, Wacker F, Vogel-Claussen J. 3D phase-resolved functional lung ventilation MR imaging in healthy volunteers and patients with chronic pulmonary disease. Magn Reson Med. 2021 Feb;85(2):912-925.
11. Kirby M, Heydarian M, Svenningsen S, et al. 3He magnetic resonance functional imaging semiautomated segmentation. Acad. Radiol. 2012;19(2):141-52
12. Thomen RP, Walkup LL, Roach, DJ, et al. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J. Cyst. Fibros. 2017;16(2):275-282
Figures