1980
Joint relaxometry-diffusion imaging and spectra of the fetal lungs.1Perinatal imaging, King's College London, London, United Kingdom, 2Centre for Medical Image Computing and Department of Computer Science, University College London, London, United Kingdom, 3Asthma UK Centre for Allergic Mechanisms in Asthma, King's College London, London, United Kingdom, 4Neonatal Unit, King’s College Hospital, London, United Kingdom, 5NIHR Biomedical Research Centre, Guy’s & St Thomas NHS Foundation Trusts and King’s College London, London, United Kingdom, 6Fetal Medicine Unit, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
Synopsis
Keywords: Data Analysis, Fetus, Prenatal lungs, non-invasive tool
We scanned the fetal lungs of healthy controls and fetuses that subsequently delivered preterm, to characterise typical and atypical lung development in-utero, with a combined T2*-diffusion protocol. We analysed our data with a joint T2*-ADC model, yielding voxelwise maps of T2* and ADC, plus calculated whole-lung T2*-ADC spectra. A reduction in both T2* and ADC values was demonstrated in the preterm cohort, potentially reflecting alterations in pulmonary development commencing in-utero. Our study demonstrates that voxelwise T2* and ADC maps, and whole-lung T2*-ADC spectra have the potential to provide additional information on fetal lung microstructure and improve detection of atypical development.Introduction
Preterm birth affects around 15 million babies around the world each year [1]. It is associated with significant postnatal pulmonary complications such as pulmonary hypoplasia and respiratory distress. Non-invasive tools can offer improved characterisation of the antenatal lung beyond 2D-measurements of lung size and basic volumetry derived from either ultrasound or MRI, which fail to provide functional information and often are hampered by their sensitivity to fetal motion. Combined diffusion-relaxation MRI has the potential to yield additional anatomical and quantitative details of distinct microstructures within the fetal lungs. Combined T2*-diffusion MRI simultaneously probes tissue microstructure and oxygenation [2] and is hence well-suited to lung characterisation. When combined with motion-corrected volumetry it may therefore help enhance the counselling in cases of fetuses at high-risk of preterm birth. In this study we scanned 55 fetal lungs with a combined T2*-diffusion protocol and hence generated mean T2* and mean ADC values and maps of the fetal lungs and evaluated their relationships with advancing gestational age. We also calculated whole-lung T2*-ADC spectra [3] and compared these between control and preterm fetal lungs.Methods
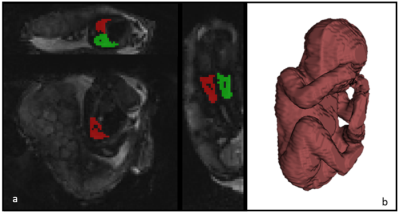
41 control fetuses (delivery >37 weeks and no sign of major pregnancy complications) and 14 fetuses delivered before 32 weeks were included in the study. All women were scanned on a clinical 3T Philips Achieva MRI scanner using a 32-channel cardiac coil, while keeping constant verbal interaction and life monitoring throughout the scan. We scanned with a combined T2*-diffusion protocol (ZEBRA [4]) that comprises multiple diffusion-weighting volumes each followed by a series of gradient echoes, hence yielding simultaneous sensitivity to T2* and diffusion properties. The diffusion encodings comprised multiple diffusion gradient directions at b = [5,10,18,25,36,50,100,200, 400, 600, 800, 1200, 1600] s/mm2 [4], and four echo times, 78, 114, 150, 186 ms, for each diffusion weighting. Further parameters were FOV = 300×320×84 mm, TR = 7 s, SENSE = 2.5, halfscan = 0.6, resolution = 3mm3. Regions-of-interest containing the lung tissue avoiding the vasculature were manually segmented (Figure 1) on the resulting images. All image data was post-processed following in-house-developed pipelines which included motion-correction. Data was analysed by fitting a joint T2*-ADC model [5] with non-linear least squares, using modified dmipy code [6] to yield voxelwise T2* and ADC values, from which the respective histograms and plots were obtained. We also fit a continuum model to the ROI-averaged data using an inverse Laplace transform [4], hence calculating a T2*-ADC spectrum for each scan.Results
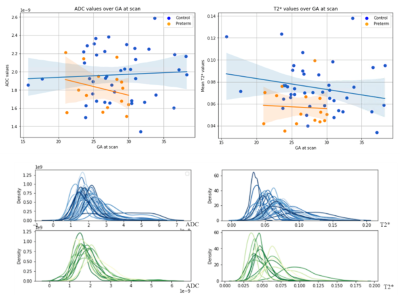
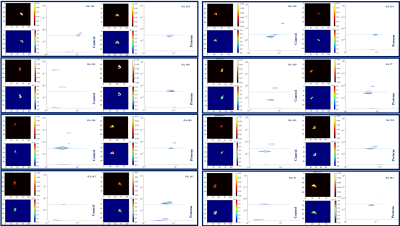
Initial results from the 14 datasets in the preterm cohort suggest a reduction in mean T2* and ADC values when plotted against the 41 control cases (Figure 2). We reveal a promising trend, with mean fetal lung T2* typically lower in preterms, although more subjects are required to reveal the strength of this effect. Visual histogram analysis supports these alterations (Figure 2). We paired 8 controls with 8 preterm cases, gestationally matched, along with their respective mean T2*-ADC maps from adapted combined T2*-ADC fit (Figure 3). We can distinguish a minimum of 2 peaks in all preterm cases equating to different ADC values and, depending on the dataset, somewhat similar T2* values. This is reflected in the associated maps in which the visually differentiable components within the lungs translate to almost parallel peaks along the x axis (T2*). Although difficult to discern with a small number of cases, it appears that the control cases exhibit widely distinctive peaks, very different ADC values, compared to the preterm cases with a less disparate spread (Figure 3).Discussion
Analysis of paired spatially matched ADC-T2* datasets of the fetal lungs demonstrate initial promise as preterm biomarkers. Histogram-based analysis revealed trends towards significance with promising lower trends in the preterm cohorts. Although in its initial stage, we have also demonstrated the potential ability of the T2*-ADC spectra to differentiate various components within the fetal lungs of both control and preterm cases for the first time. The appearance of a minimum of two peaks noticeably separated by diffusivity in all the control cases (Figure 3) is consistent with the complexity of prenatal lung development with distinct tissue compartments and is reflected in the associated maps. This separation is less apparent in the preterm cases which could indicate an insult happening before the premature birth itself and could reflect altered metabolic activity during early lung development in those fetuses. Additional T2*-ADC spectra for a wider GA range and increased case numbers may also help to reveal some new trends.Conclusion
This study provides original motion-corrected morphological and functional assessment of the antenatal fetal lung, comparing fetuses that delivered at term with those who subsequently delivered preterm using advanced non-invasive post-processing techniques. These are preliminary results, but we can confirm that the combined T2*-diffusion acquisition combined with mono-exponential modeling is a feasible technique for generating insights into the developing lungs in-utero. Future work is needed but generating T2*-ADC spectra within the prenatal lungs paves the way for future in-depth tissue analysis and prenatal pulmonary evaluation especially relevant in fetuses at high-risk of preterm birth.Acknowledgements
This work was supported by supported by Health Education England (HEE) / National Institute for Health Research (NIHR) for this research project, the NIH Human Placenta Project grant 1U01HD087202-01 (Placenta Imaging Project (PIP)), Wellcome Trust Collaboration in Science grant [WT201526/Z/16/Z], a Sir Henry Wellcome Fellowship, a UKRI FL fellowship and by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
[1] Blencowe H, Cousens S, Oestergaard M, Chou D, Moller AB, Narwal R, Adler A, Garcia CV, Rohde S, Say L, Lawn JE. National, regional and worldwide estimates of preterm birth. The Lancet, June 2012. 9;379(9832):2162-72. Estimates from 2010.
[2] Slator, P. J., et al. (2019). "Combined diffusion-relaxometry MRI to identify dysfunction in the human placenta." Magn Reson Med 82(1): 95-106.
[3] English, A. E., et al. (1991). "Quantitative Two-Dimensional time Correlation Relaxometry." Magnetic Resonance in Medicine 22(2): 425-434.
[4] Hutter, J., et al. (2018). "Integrated and efficient diffusion-relaxometry using ZEBRA." Scientific Reports 8(1): 15138.
[5] Slator, P. J., et al. (2019). "Combined diffusion-relaxometry MRI to identify dysfunction in the human placenta." Magn Reson Med 82(1): 95-106.
[6] Fick, R. H. J., et al. (2019). "The Dmipy Toolbox: Diffusion MRI Multi-Compartment Modeling and Microstructure Recovery Made Easy." Frontiers in Neuroinformatics 13.
Figures