1972
The Association of Peritumoral Vasculature Radiomics of Lung Cancer Brain Metastasis with Patient Outcome after Radiosurgery1National Yang Ming Chiao Tung University, Taipei, Taiwan, Taipei, Taiwan, 2Department of Neurosurgery, Neurological Institute, Taipei Veteran General Hospital, Taipei, Taiwan, Taipei, Taiwan, 3Department of Radiology, Taipei Veteran General Hospital, Taipei, Taiwan, Taipei, Taiwan, 4Department of Medical Imaging, Cheng-Hsin General Hospital, Taipei, Taiwan, Taipei, Taiwan
Synopsis
Keywords: Tumors, Radiotherapy
Gamma Knife radiosurgery (GKRS) is a first-line treatment for brain metastases (BMs) from non-small cell lung cancer (NSCLC). The convolutedness, leakiness and disorganized structure of the peritumoral vasculature have been suggested to be related to the treatment resistance of the tumor. In this study, radiomic features that measure the morphology and spatial organization of peritumoral vasculature were extracted from pre-GKRS MRI. These features were applied to predict overall survival after GKRS in NSCLC-BM patients. We suggested that peritumoral vasculature radiomics could facilitate patient management by identifying potential benefits of GKRS.Background and Purpose
Brain metastasis (BM) from non-small cell lung cancer (NSCLC) is a common type of malignant brain tumor. Because of the promising tumor control rate, Gamma Knife radiosurgery (GKRS) has been one of the first-line treatments for BMs [1]. Peritumoral vasculature could reflect tumor microenvironment and may be associated with treatment resistance. Tumor-associated vascular features extracted from structural images showed potential to predict treatment response in cancers [2]. In this study, we aimed to extract radiomic features of peritumoral vasculature from pre-GKRS MRI and further combined these features with clinical information to investigate the feasibility of the overall survival (OS) prediction in NSCLC-BM patients after GKRS treatment.Materials and Methods
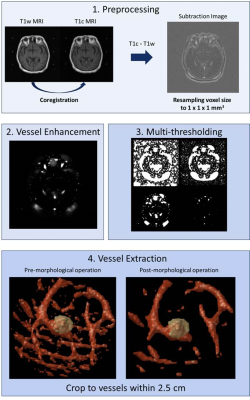
We retrospectively collected MRI and clinical data of 230 patients with BMs from NSCLC who received GKRS treatment. The inclusion criteria included: 1) pathological diagnosis of NSCLC by lung biopsy or surgery; 2) diagnosis of BMs confirmed by MRI; and 3) available clinical and MRI follow-up after GKRS. The clinical characteristics of included BM patients are listed in Table 1. Furthermore, several prognostic clinical features (status of epidermal growth factor receptor; Karnofsky performance status, KPS; existence of extracranial metastasis; therapeutic effect of original NSCLC; number of BMs; volume of BMs; additional treatment) were collected in this study [3].All the patients underwent the MRI examination before GKRS, including contrast-enhanced T1-weighted (T1c) and T1-weighted (T1w) images. Several preprocessing steps were applied to MRIs to improve the reliability of vasculature radiomics analysis. The image resolution was first adjusted by resampling voxel size to an isotropic resolution of 1 mm3 for each MRI modality. Registrations of T1w to T1c images were performed by a six-parameter rigid body transformation and mutual information algorithm. The subtracted images were calculated based on the difference between T1c and T1w images to enhance the signal of tissues perfused with contrast agent. A vessel enhancement filter was then applied to the subtraction images [4] followed by the Otsu’s thresholding method to extract vasculature signals from background. Finally, morphologic operations for removing small and spherical objects, were applied to eliminate noise and refine the vascular structure. The tumor region was defined by radiation oncologists and reviewed by a neuro-radiologist for the GKRS treatment planning based on T1c images. The region of peritumoral vasculature was defined as the surrounding voxels (approximately 2.5 cm) outside the tumor boundary. The workflow of image processing is displayed in Figure 1. Overall 91 peritumoral vasculature radiomic features, including morphology and spatial organization, were extracted.
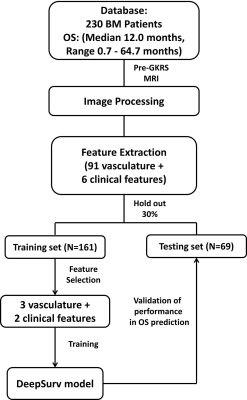
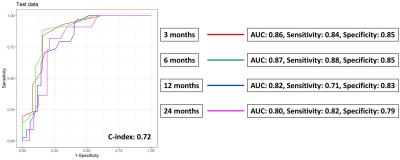
To identify key features for model training, univariate Cox proportional hazards models and Chi-squared tests were applied on the 70% of patients (training set) for selecting of vasculature radiomic and clinical features, respectively. A DeepSurv survival network (4 hidden layers, 8 nodes, and 1000 epochs) with the selected features as inputs was then trained to predict OS in patients with NSCLC-BM [5]. The model performance was evaluated based on the remaining 30% of patients (testing set). The flowchart of feature selection and model training is shown in Figure 2. Time-dependent receiver operating characteristic (ROC) curves, index of concordance (C-index), sensitivity, and specificity were estimated to assess the prediction performance at 4 different time-points (3 months, 6 months, 12 months and 24 months).
Results
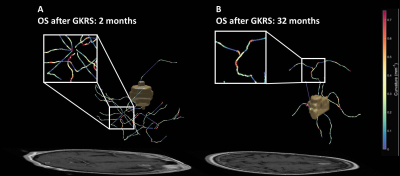
A total of 3 vasculature radiomics exhibited significant correlation (p<0.05) with OS after GKRS. These features describe the number of branches of the peritumoral vasculature and the vessel’s rotation with respect to the tumor. For clinical features, the usage of tyrosine kinase inhibitors and KPS were selected. Time-dependent ROC curves at different survival time are showed in the Figure 3. The constructed DeepSurv model achieved a C-index of 0.72 and area under ROC curves (AUC) of 0.86, 0.87, 0.82 and 0.80, sensitivities of 0.84, 0.88, 0.71 and 0.82, and specificities of 0.85, 0.85, 0.83 and 0.79 for the OS prediction at 3, 6, 12 and 24 months, respectively. The visualization of the peritumoral vessel distribution is shown in Figure 4. The case with poor prognosis showed greater curvature and number of branches than the case with good prognosis in the peritumoral vasculature. Our results showed that the peritumoral vasculature radiomic features were feasible to predict OS of BM patients.Conclusions
In this study, we suggested that the peritumoral vasculature features extracted from pre-treatment MRI could predict the OS in NSCLC-BM patients after GKRS. Our findings indicated that the distribution and structure of peritumoral vasculature provided valuable information for patient prognosis.Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-010-022-MY3) and AICS, Asustek Computer Incorporation, Taiwan (110J042).References
1. Serizawa, T., et al., Gamma knife radiosurgery for metastatic brain tumors from lung cancer: a comparison between small cell and non—small cell carcinoma. Journal of neurosurgery, 2002. 97(Supplement 5): p. 484-488.
2. Braman, N., et al., Novel Radiomic Measurements of Tumor-Associated Vasculature Morphology on Clinical Imaging as a Biomarker of Treatment Response in Multiple Cancers. Clinical Cancer Research, 2022. 28(20): p. 4410-4424.
3. Sperduto, P.W., et al., Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncology, 2017. 3(6): p. 827-831.
4. Frangi, A.F., et al. Multiscale vessel enhancement filtering. in International conference on medical image computing and computer-assisted intervention. 1998. Springer.
5. Katzman, J.L., et al., DeepSurv: personalized treatment recommender system using a Cox proportional hazards deep neural network. BMC medical research methodology, 2018. 18(1): p. 1-12.
Figures