1971
A clini-radiomics model based magnetic resonance imaging for differentiating fibroblastic from non-fibroblastic meningioma1Lanzhou University Second Hospital, LanZhou, China
Synopsis
Keywords: Tumors, Machine Learning/Artificial Intelligence
This study evaluated the feasibility of noninvasive preoperative differentiation fibroblastic meningioma (FM) from non-fibroblastic meningiomas (nFM) based a clini-radiomics model. A total of thirteen radiomics features were included after Selectpercentile and Lasso feature screening. Our results showed that Random forest is the most efficient among the six radiomics models in differentiating FM from nFM, and the diagnostic efficacy of clini-radiomics models in training and validation group is further improved. Therefore, we believe that the clini-radiomics model is of great value in noninvasive preoperative differentiation of FM and nFM and contributes to the selection of individualized treatment options for meningioma patients.Introduction
Introduction: Meningiomas are primary central nervous system tumors that accounting for 39% of all brain tumors and the incidence is increasing annually1. Surgical resection is currently the main treatment for WHO grade I meningioma2, however, treatment options for different subtypes of meningiomas vary according to their tissue composition and consistency. Several studies3,4 have reported that the consistency of meningioma is one of the key factors in determining the difficulty of surgery. Soft-textured meningiomas that are easily aspirated5, FM are usually harder to remove and take longer to operate, and require the use of an ultrasound aspirator to extract6. Therefore, preoperative discrimination of FM is critical for the selection of surgical options and reduction of potential complications. radiomics provides a non-invasive, comprehensive quantification of tumor phenotypes by extracting numerous microscopic features that the naked eye cannot recognize, reflecting tumor heterogeneity and pathophysiological information7. To knowledge, no studies have been conducted on the clini-radiomics models to differentiate FM from nFM. Therefore, the purpose of this study to investigate the value of a clini-radiomics model based magnetic resonance imaging (MRI) for differentiating FM from nFM.Method
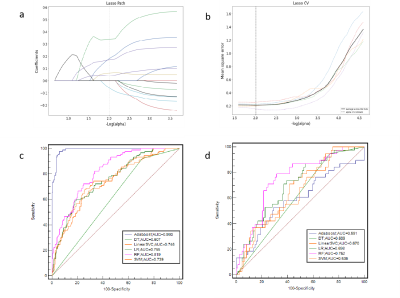
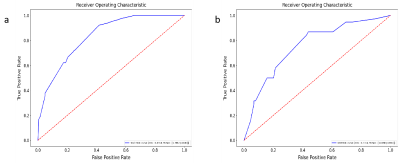
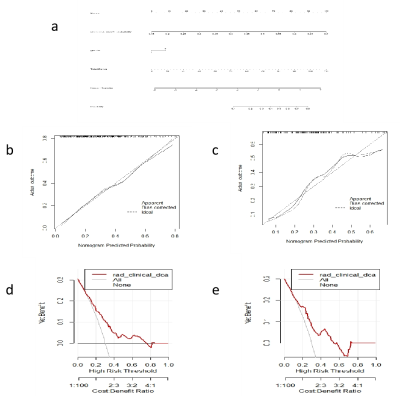
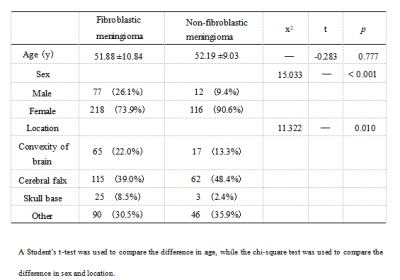
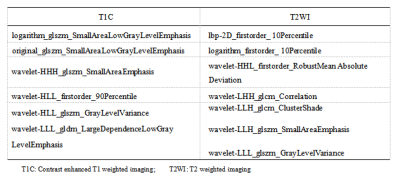
Methods: This study included 423 patients (128 FM and 295 nFM) who underwent T2-weighted imaging (T2WI) and contrast-enhanced T1-weighted imaging (T1C) on Siemens Verio 3.0T superconducting MRI scanner (Siemens, Germany) that were randomly categorized into training (n=296) and validation (n=127) groups at a 7:3 ratio. The Selectpercentile and LASSO were used to selected the highly correlated features from 3376 radiomics features. Different classifiers were used to train and verify the model. The receiver operating characteristic (ROC) curves, ACC, SEN, and SPE were drawn to evaluate the performance. The optimal radiomics model was selected, calibration curves and decision curve analysis were used to verify the clinical utility and consistency of the nomogram constructed from the radiomics features and clinical factors.Results
Results: There were thirteen radiomic features selected from T1C and T2WI after dimensionality reduction. The prediction performance of RF radiomics model is slightly lower than that of the clini-radiomics model. The area under the curve (AUC), SEN, SPE, and ACC of the clini-radiomics model training set are 0.836 (95% confidence interval [CI], 0.795-0.878), 0.922, 0.583, and 0.686; the AUC, SEN, SPE, and ACC of the validation set were 0.756 (95% CI, 0.660-0.846), 0.816, 0.596, and 0.661, respectively.Discussion
Discussion:In this study, FM and nFM were differentiated noninvasively preoperatively based on a clini-radiomics model. The results suggest that the diagnostic efficacy and sensitivity of the cli-radiomics model with clinical features (sex) were improved compared with those of the RF radiomics model.The calibration curve and Hosmer-Lemeshow test further confirmed the good agreement between the actual differentiation of FM and nFM and the prediction of meningioma subtypes using the cli-radiomics model (p=0.237 and p=0.136, respectively). Analysis of the decision curve provides an important basis for preoperative diagnosis and surgical decision-making by radiologists and clinicians.Conclusion
Conclusion: The diagnostic efficacy of the clini-radiomics model of fibroblastic meningioma and non-fibroblastic meningioma was better than that of the radiomics prediction model alone, and can be used as a potential tool for clinical surgical planning and evaluation of patient prognosis.Acknowledgements
We are greatly indebted to all patients, doctors, and statistical consultants who were involved in our study.References
1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol, 2021. 23(12 Suppl 2): p. iii1-iii105.
2. Kim L. A narrative review of targeted therapies in meningioma. Chin Clin Oncol, 2020. 9(6): p. 76.
3. Zhai Y, Song D, Yang F, Wang Y, Jia X, Wei S, Mao W, Xue Y, Wei X. Preoperative Prediction of Meningioma Consistency via Machine Learning-Based Radiomics. Front Oncol, 2021. 11: p. 657288.
4. Cepeda S, Arrese I, García-García S, Velasco-Casares M, Escudero-Caro T, Zamora T, Sarabia R. Meningioma Consistency Can Be Defined by Combining the Radiomic Features of Magnetic Resonance Imaging and Ultrasound Elastography. A Pilot Study Using Machine Learning Classifiers. World Neurosurg, 2021. 146: p. e1147-e1159.
5. Winter F, Furtner J, Pleyel A, Woehrer A, Callegari K, Hosmann A, Herta J, Roessler K, Dorfer C. How to predict the consistency and vascularity of meningiomas by MRI: an institutional experience. Neurol Res, 2021. 43(8): p. 693-699.
6. Miyoshi K, Wada T, Uwano I, Sasaki M, Saura H, Fujiwara S, Takahashi F, Tsushima E, Ogasawara K. Predicting the consistency of intracranial meningiomas using apparent diffusion coefficient maps derived from preoperative diffusion-weighted imaging. J Neurosurg, 2020: p. 1-8.
7. Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, Forster K, Aerts HJ, Dekker A, Fenstermacher D, Goldgof DB, Hall LO, Lambin P, Balagurunathan Y, Gatenby RA, Gillies RJ. Radiomics: the process and the challenges. Magn Reson Imaging, 2012. 30(9): p. 1234-48.
Figures