1960
Prediction of brain invasion in meningioma by preoperative MRI based on U-NET and DenseNet convolutional neural network1Radiology Department, Shenzhen Second People's Hospital, Shenzhen, China, 2Shenzhen University, Shenzhen, China, 3Philips Healthcare, Guangzhou, China, 4Shenzhen Second People's Hospital, Shenzhen, China, 5The First Affiliated Hospital of Jinan University, Guangzhou, China
Synopsis
Keywords: Tumors, Tumor, meningioma
The new guidelines suggest that brain invasion is not a unique feature of malignant meningiomas, but may be a pathologic condition of benign meningiomas with a potential risk of recurrence. However, maximum preservation of normal brain tissue is essential for surgery. Accurate preoperative MRI evaluation of meningioma brain invasion is expected to solve the dilemma faced by neurosurgeons.Objective
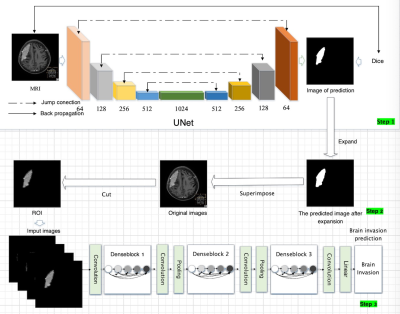
The new guidelines suggest that brain invasion is not a unique feature of malignant meningiomas, but may be a pathologic condition of benign meningiomas with a potential risk of recurrence. However, maximum preservation of normal brain tissue is essential for surgery. Accurate preoperative MRI evaluation of meningioma brain invasion is expected to solve the dilemma faced by neurosurgeons.In this study, U-Net segmentation model was trained for automatic segmentation of meningiomas, and the region of interest was postprocessed according to the diagnostic requirements to highlight the edge information. Then the DenseNet121 classification model was trained to diagnose meningiomas brain invasion to explore the predictive value of preoperative MRI based on deep learning for brain invasion.
Materials and methods
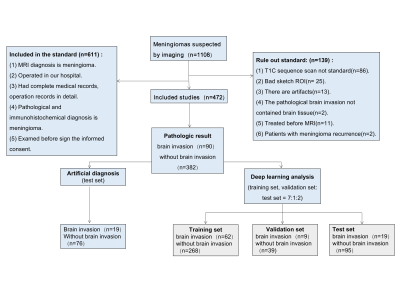
A retrospective analysis was performed on 472 patients with meningioma who underwent MRI examination in a hospital from January 2006 to December 2020. Thin-slice T1 weighted image contrast enhancement sequence was used as the study data set and randomly divided into training set, validation set and test set at 7:1:2 ratio,and the positive ratio was kept the same in all data sets. The study set pathological diagnostic as the gold standard.After data was prepared, U-Net was used to automatically segment 472 cases of meningioma images, predicting the region of interest(ROI)of meningiomas in test set, analyzing the Dice coefficient, positive predictive value and sensitivity of U-Net, and making comparative analysis with other networks. After segmentation, to compare the effects of different regions of interest, which were post-processed by different methods,on the classification of brain invasion. Then, the classical DenseNet121 network was used to evaluate the performance of the classification task for brain invasion, and the accuracy, positive predictive value, negative predictive value, sensitivity and specificity were calculated. To reduce randomness, five tests were performed and the average data was compared to other classification networks. Finally, the effectiveness comparison and consistency analysis between deep learning method and manual diagnosis method are carried out. ROC curves were made and the differences were compared by Delong Test.Result
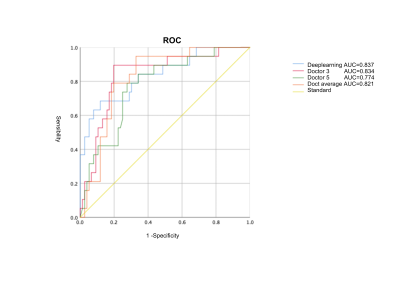
1.1. Pathological ResultsThere were 90 brain invasion cases in 472 meningiomas. Among them, 49 cases were identified as BIOB, and 41 cases were identified as WHO grade 2 and 3 meningiomas on morphology.1.2. Analysis of diagnostic results of brain infiltration in five doctorsThe Kappa/ICC of the features of five doctors' diagnoses ranged from 0.702 to 0.957, showing uneven consistency. Among the five doctors, doctor 3 (8 years experience) had the best diagnostic efficiency. ROC curve showed that the AUC was 0.834 (0.734~0.935), the sensitivity was 0.894, the specificity was 0.815, the positive predictive value was 0.548, the negative predictive value was 0.968, and the accuracy was 0.831. The AUC of mean diagnostic efficiency of radiologists was 0.821 (0.731~0.910). The sensitivity, specificity, positive predictive value, negative predictive value and accuracy were 0.789, 0.829, 0.536, 0.940 and 0.831 respectively. There was significant difference in AUC between Doctor 3 and Doctor 5 (P=0.022).
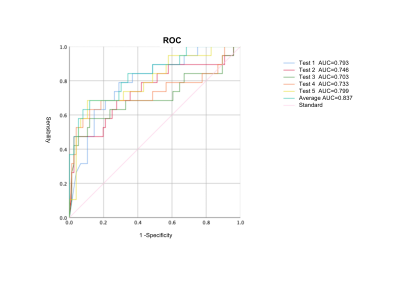
1.3. Performance evaluation of U-Net in the segmentation task of meningiomaIn the segmentation task of meningiomas, the results of U-Net in the test set were: Dice:0.8228, positive predictive value:0.839, sensitivity:0.857. The prediction of region of interest by U-Net is more accurate than the performance of DANet,PraNet and ResUNet networks.
1.4. Post-processing of thearea of interestThe best performance for classification diagnosis of brain invasion was the post-processing image of the whole tumor area of interest, with an accuracy of 0.854, positive predictive value of 0.501, negative predictive value of 0.963, sensitivity of 0.695, and specificity of 0.882. However, the segmentation image with only tumor boundary was close to the image with tumor internal information andboundary, with accuracy of 0.833, positive predictive value of 0.602, negative predictive value of 0.900, sensitivity of 0.600, and specificity of 0.892.1.5. Performance evaluation of DenseNet121 classification networkDenseNet performed better than ResNet and ShuffleNet V2 in the classification of meningioma brain invasion. The average results of the five DenseNet models on the test set showed as follows: accuracy:0.854,sensitivity:0.695,specificity:0.882, positive predictive value:0.501,negative predictive value:0.963.1.6. Comparison between deep learning methods and artificial resultsDeep learning model has a higher diagnostic efficiency for brain invasion than an experienced radiologist (ACC: 0.854 VS 0.831), but there was no statistically significant difference in ROC curve between them (P=0.964), and that of the average efficacy of radiologists (P=0.743), and that of a doctor with less experience (P=0.034).
Conclusion
In conclusion, MRI-based deep learning model is a new non-invasive method that can predict the risk of brain invasion in meningiomas, and may become a potentially non-invasive tool to help radiologists to improve the efficiency and play an important role in daily clinical work.The information of tumor margin plays an important role in diagnosing brain invasion of meningiomas.Acknowledgements
No acknowledgement found.References
1.Behling F, Hempel J M, Schittenhelm J. Brain Invasion in Meningioma-A Prognostic Potential Worth Exploring[J]. Cancers (Basel). 2021, 13(13).
2.Bulleid L S, James Z, Lammie A, et al. The effect of the revised WHO classification on the incidence of grade II meningioma[J]. Br J Neurosurg. 2020, 34(5): 584-586.
3.Behling F, Fodi C, Gepfner-Tuma I, et al. CNS Invasion in Meningioma-How the Intraoperative Assessment Can Improve the Prognostic Evaluation of Tumor Recurrence[J]. Cancers (Basel). 2020, 12(12).
4.Adeli A, Hess K, Mawrin C, et al. Prediction of brain invasion in patients with meningiomas using preoperative magnetic resonance imaging[J]. Oncotarget. 2018, 9(89): 35974-35982.5.
5.Zhang J, Yao K, Liu P, et al. A radiomics model for preoperative prediction of brain invasion in meningioma non-invasively based on MRI: A multicentre study[J]. EBioMedicine. 2020, 58: 102933.[56] Doddamani R S, Meena R K, Sawarkar D. Ambiguity in the Dural Tail Sign on MRI[J]. Surg Neurol Int. 2018, 9: 62.
6.Niu J, Zhang S, Ma S, et al. Preoperative prediction of cavernous sinus invasion by pituitary adenomas using a radiomics method based on magnetic resonance images[J]. Eur Radiol. 2019, 29(3): 1625-1634.
7.Zwirner K, Paulsen F, Schittenhelm J, et al. Integrative assessment of brain and bone invasion in meningioma patients[J]. Radiat Oncol. 2019, 14(1): 132.
8. Ostrom Q T, Gittleman H, Xu J, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013[J]. Neuro Oncol. 2016, 18(suppl_5): v1-v75.
9. Chen C, Cheng Y, Xu J, et al. Automatic Meningioma Segmentation and Grading Prediction: A Hybrid Deep-Learning Method[J]. J Pers Med. 2021, 11(8).
10. Yun S, Koh J M, Lee K S, et al. Expression of c-MET in Invasive Meningioma[J]. J Pathol Transl Med. 2015, 49(1): 44-51.
11. Vranic A, Popovic M, Cör A, et al. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients[J]. Neurosurgery. 2010, 67(4): 1124-1132.
12. Spille D C, Heß K, Sauerland C, et al. Brain Invasion in Meningiomas: Incidence and Correlations with Clinical Variables and Prognosis[J]. World Neurosurg. 2016, 93: 346-354.
13. Butts A M, Weigand S, Brown P D, et al. Neurocognition in individuals with incidentally-identified meningioma[J]. J Neurooncol. 2017, 134(1): 125-132.
14.Krivoshapkin A L, Sergeev G S, Kalneus L E, et al. New Software for Preoperative Diagnostics of Meningeal Tumor Histologic Types[J]. World Neurosurg. 2016, 90: 123-132.
15. Masalha W, Heiland D H, Delev D, et al. Survival and Prognostic Predictors of Anaplastic Meningiomas[J]. World Neurosurg. 2019, 131: e321-e328.
16. Lemée J M, Joswig H, Da B M, et al. WHO grade I meningiomas: classification-tree for prognostic factors of survival[J]. Neurosurg Rev. 2020, 43(2): 749-758.
17. Yu J, Chen F F, Zhang H W, et al. Comparative Analysis of the MRI Characteristics of Meningiomas According to the 2016 WHO Pathological Classification[J]. Technol Cancer Res Treat. 2020, 19: 1079250935.
18. Karsy M, Guan J, Cohen A, et al. Medical Management of Meningiomas: Current Status, Failed Treatments, and Promising Horizons[J]. Neurosurg Clin N Am. 2016, 27(2): 249-260.
19. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas[J]. Lancet Oncol. 2016, 17(9): e383-e391.
20. Birzu C, Peyre M, Sahm F. Molecular alterations in meningioma: prognostic and therapeutic perspectives[J]. Curr Opin Oncol. 2020, 32(6): 613-622.
21. Simis A, Pires D A P, Leite C C, et al. Peritumoral brain edema in benign meningiomas: correlation with clinical, radiologic, and surgical factors and possible role on recurrence[J]. Surg Neurol. 2008, 70(5): 471-477, 477.
22. Maiuri F, Donzelli R, Pagano S, et al. The Management of the Venous Sinuses During Surgery for Posterior Fossa Meningiomas[J]. World Neurosurg. 2019, 125: 357-363.
23. Zhu Y, Man C, Gong L, et al. A deep learning radiomics model for preoperative grading in meningioma[J]. Eur J Radiol. 2019, 116: 128-134.
24. 朱永北. 基于定量影像组学的脑肿瘤的分级预测[D]. 哈尔滨理工大学, 2019.
25. Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology[J]. Nat Rev Cancer. 2018, 18(8): 500-510.
26.Jo T, Nho K, Saykin A J. Deep Learning in Alzheimer's Disease: Diagnostic Classification and Prognostic Prediction Using Neuroimaging Data[J]. Front Aging Neurosci. 2019, 11: 220.