1958
Predicting Adult-type Diffuse Gliomas Grade: A Comparison of Four Radiomics-based Diffusion Prediction Models
Peng Wang1,2, Jinlong He1, Qiong Wu1, Shenghui Xie1, Lixin Weng2, Shaoyu Wang3, Huapeng Zhang3, Yang Song3, and Yang Gao1
1Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China, 2Inner Mongolia Medical University, Hohhot, China, 3Siemens Healthineers, Shanghai, China
1Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China, 2Inner Mongolia Medical University, Hohhot, China, 3Siemens Healthineers, Shanghai, China
Synopsis
Keywords: Tumors, Diffusion/other diffusion imaging techniques
Application of MRI diffusion models can aid in the formulation of logical treatment strategies by non-invasively predicting gliomas pathogenic grade. However, it appears that the thorough comparison of diffusion models has not been addressed or has only been partially addressed. The objective of this study was to compare the potential clinical uses of four radiomics-based diffusion prediction models for predicting adult-type diffuse gliomas grade. According to the findings, the relatively simple model (i.e. diffusion tensor imaging) had the same potential for clinical use than the more sophisticated diffusion models. This suggests that additional point-to-point research is required.Background and Purpose
There isn't yet a study that compares the potential of several diffusion models to forecast the grade of adult-type diffuse gliomas. We created four radiomics-based diffusion models and compared their potential for use in clinical settings.Methods
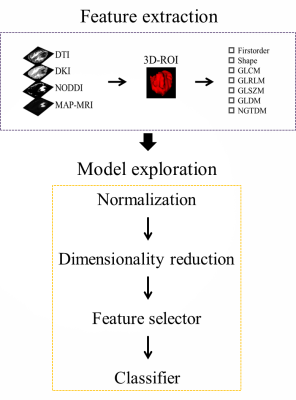
A total of 103 participants (mean age, 52 years; range, 21-77; 54 [52%] male) with adult-type diffuse gliomas were included in this prospective analysis. Participants in the study included 46 low-grade gliomas (Central Nervous System WHO Grade 2-3) and 57 high-grade gliomas (Central Nervous System WHO Grade 4). Every participant got preoperative MRI using a 3T scanner (MAGNETOM Skyra,Siemens Healthineers, Erlangen, Germany). The scanning sequences included conventional structural sequence and diffusion spectrum magnetic resonance imaging (DSI) sequence. The DSI sequence was obtained in the axial plane using a half q-space Cartesian grid sampling procedure under the following parameters: TR/TE = 7000/107 ms, FOV = 260 mm × 260 mm, GRAPPA = 2, layer thickness = 3.0 mm, voxel size = 2.2× 2.2× 3.0mm3, 50 layers and maximum B value = 3000 s/mm2. All DSI raw data were processed using the NeuDiLab software developed in-house based on the open resource tool DIPY (Difusion Imaging in Python, https://dipy.org/) [1]. After preprocessing the original diffusion images, 2782 radiomics features were extracted along with 25 quantitative parameters from four different diffusion models, including three more advanced models (diffusion kurtosis imaging, neurite orientation dispersion and density imaging, and mean apparent propagation diffusion MRI) and one relatively simple model (diffusion tensor imaging). FeAture Explorer (FAE v0.5.2, https://github.com/salan668/FAE) [2] was used to generate the radiomics models, and various pipelines were used to select the optimal model. By using the leave-one-out cross-validation method, hyperparameters were identified. Next, accuracy was assessed using ROC and PR curves, and the Delong test was employed to analyze model differences. The potential clinical application possibilities were examined using calibration curves and decision curve analysis.Results
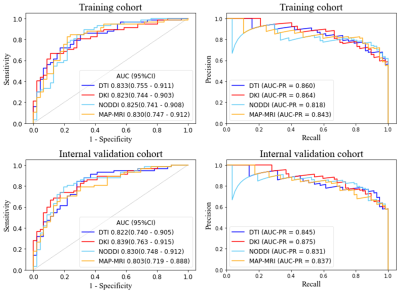
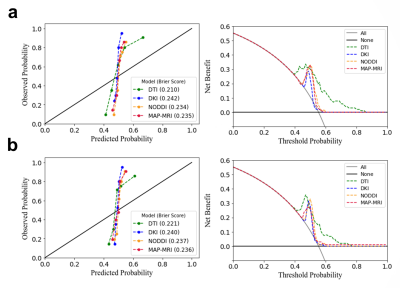
The AUC ranged from 0.818 to 0.864 for the four radiomics-based diffusion prediction models. In the training or internal validation sets, there were no differences in the AUC between four models (p<.05). The calibration curves demonstrate that for these four models, there is still a large difference between the predicted values and the true state distribution. And according to the decision curve analysis, the clinical value of the four prediction models appeared to be comparable.Discussion and Conclusion
Our study discovered that the four radiomics-based diffusion prediction models had comparable diagnostic efficacy and clinical benefit, and that the diagnostic models created by the advanced diffusion model did not have as high diagnostic efficacy as expected, compared to the relatively simple diffusion model. Similar results were achieved in a prior investigation [3]. This shows that there is still some distance between the theoretical benefits of advanced models and their actual clinical implementations. It is still anticipated that more noninvasive diffusion imaging will be used as a new imaging marker to enhance clinical diagnosis.Acknowledgements
This work was supported by the Inner Mongolia Autonomous Region Science and Technology Plan Project (2019GG047). The authors gratefully acknowledge the essential contributions of the research staff of Affiliated Hospital of Inner Mongolia Medical University.References
1. Wang P, Weng L, Xie S, et al. Primary application of mean apparent propagator-MRI diffusion model in the grading of diffuse glioma. Eur J Radiol 2021;138:109622.2.2.
2. Song Y, Zhang J, Zhang YD, et al. FeAture Explorer (FAE): A tool for developing and comparing radiomics models. PLoS One. 2020;15(8):e0237587. Published 2020 Aug 17.3.3.
3. Gao A, Zhang H, Yan X, et al. Whole-Tumor Histogram Analysis of Multiple Diffusion Metrics for Glioma Genotyping. Radiology. 2022;302(3):E16. doi:10.1148/radiol.219034
DOI: https://doi.org/10.58530/2023/1958