1952
Multi-parametric Z-spectral MRI of stroke patients1Guangzhou First People's Hospital, South China University of Technology, Guangzhou, China, 2Philips Healthcare, Guangzhou, China, 3Philips Healthcare, Shanghai, China
Synopsis
Keywords: Stroke, Ischemia, ischemic stroke, Multi-parametric Z-spectral MRI, lesions
Multi-parametric Z-spectral MRI has been successfully implemented in vivo. In this study, multi-contrast Z-spectra fitted with four Lorentzian functions were acquired from eighteen ischemic stroke patients. We found that this novel method could differentiate lesions from normal brain tissues, and multiple Z-spectral contrasts showed a satisfying correlation with the apparent diffusion coefficient (ADC). Multi-parametric Z-spectral MRI could specifically characterize the metabolic alterations of ischemic brain tissues in stroke patients.Introduction
The Z-spectral fitting method with a set of Lorentzian functions has been implemented for brain tumors to quantify each Z-spectral contribution separately.1, 2 In this study, we attempt to perform multi-parametric Z-spectral MRI of ischemic stroke patients and evaluate the potential value of multiple Z-spectral parameters imaging for detecting metabolic changes at the molecular level and physiopathologic changes of brain tissues due to ischemia.Methods
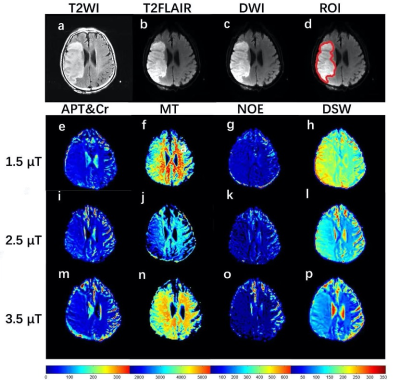
Three sets of Z-spectra data with different saturation power () of 1.5, 2.5, and 3.5 were acquired from eighteen ischemic stroke patients respectively. Multiple contrasts contributing to Z-spectra, corresponding to the combined effect of amide proton transfer (APT) and a +2 ppm chemical exchange saturation transfer (CEST) peak (APT&Cr), semi-solid magnetization transfer contrast (MT), aliphatic nuclear overhauser effect (NOE), and direct saturation of water (DSW) were fitted with the summation of four Lorentzian functions. Each Z-spectral contrast was quantified with the voxel-wise fitting and compared between the ischemic brain tissues and contralateral normal white matter (CNWM). The correlations between multiple Z-spectral metrics and the apparent diffusion coefficient (ADC) were analyzed as well.Results
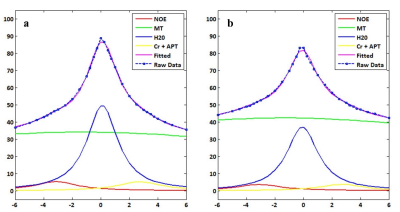
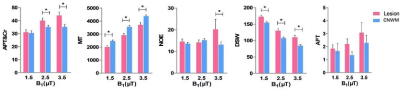
Figure 1 demonstrates Z-spectral fitting curves from representative infarct brain tissues (Lesion) and CNMW at B1 of 3.5 μT. Increased APT&Cr, NOE, DSW, and decreased MT were observed in ischemic lesions compared to CNWM (Figure 2, 3). Additionally, APT&Cr showed a good positive correlation with ADC, MT showed a good negative correlation with ADC, and the correlations of DSW and NOE with ADC were different among varying B1. However, the conventional CEST effect based on asymmetrical analysis could neither differentiate the lesions from CNWM nor show a correlation with ADC.Discussion
In the study, multi-parametric Z-spectral MRI detected ischemic lesions with elevated APT&Cr, NOE, and DSW, and a decreased MT signal. Multiple Z-spectral contrasts showed a satisfying correlation with ADC. The accuracy of the Cr fit is debatable given that the APT image contrast originates from various amide proton populations that are centered on slightly different resonances, and other potential metabolites from proteins, peptides, and amino acids would also contribute to the +2 ppm signal, as well as due to the coalescence effects between the fast exchanging pools and water pool.3,4 Therefore, we combinedly analyzed the two close ppm of +3.5 and +2, namely APT&Cr in the current study. Increased APT&Cr may be predominately due to a shift from acidosis to alkalosis in subacute ischemic stroke. Considering the fitted Cr signal may be contaminated by the broad glutamate CEST signal, DSW, and MT effect, the CEST signal at +2 ppm in brain tissues should be interpreted with caution. The increased DSW signal in ischemic lesions might be caused by cytotoxic edema, vasogenic edema, necrosis, and cystic lesions during the period of ischemia. MT reflects the interaction between the semi-solid macromolecules and the bulk water. Those immobile macromolecules in protein matrices and cell membranes of brain tissues suffered destruction after ischemia. Hence, the value of MT may be reduced in ischemia lesions. The NOE signal has been thought to arise from mobile proteins, peptides, lipids, and restricted metabolites through cross-relaxation, but the NOE contrast has not been interpreted deeply in vivo due to the influence of multi-competing effects.5 Z-spectral contrasts show a good correlation with ADC. This indicates that multiple Z-spectral contrasts have the potential for assessing metabolic changes of ischemia, and may provide supplemental information to existing MR protocols when we further study the pathophysiologic mechanism, progress, and outcome of ischemic stroke.Conclusion
Multi-parametric Z-spectral MRI could be used to specifically define metabolic changes in ischemic brain tissues, making it potentially a viable endogenous noninvasive imaging approach for monitoring stroke patients.)Acknowledgements
No acknowledgement found.References
1. Cai, K., Singh, A., Poptani, H., Li, W., Yang, S., Lu, Y., Hariharan, H., Zhou, X. J., Reddy, R. CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed, 2015,28(1):1-08.
2. Su, C., Xu, S., Lin, D., He, H., Chen, Z., Damen, F. C., Ke, C., Lv, X., Cai, K. Multi-parametric Z-spectral MRI may have a good performance for glioma stratification in clinical patients. Eur Radiol, 2022,32(1):101-11.
3. Zhang, X. Y., Wang, F., Li, H., Xu, J., Gochberg, D. F., Gore, J. C., Zu, Z. CEST imaging of fast exchanging amine pools with corrections for competing effects at 9.4 T. NMR Biomed, 2017,30(7).
4. Zhang, X. Y., Xie, J., Wang, F., Lin, E. C., Xu, J., Gochberg, D. F., Gore, J. C., Zu, Z. Assignment of the molecular origins of CEST signals at 2 ppm in rat brain. Magn Reson Med, 2017,78(3):881-87.
5. Li, H., Zu, Z., Zaiss, M., Khan, I. S., Singer, R. J., Gochberg, D. F., Bachert, P., Gore, J. C., Xu, J. Imaging of amide proton transfer and nuclear Overhauser enhancement in ischemic stroke with corrections for competing effects. NMR Biomed, 2015,28(2):200-09.
Figures