1948
Altered static and dynamic spontaneous neural activity in patients with ischemic pontine stroke
Xin Wang1, Caihong Wang1, Jingchun Liu2, Jun Guo3, Peifang Miao1, Ying Wei1, Kaiyu Wang4, Jingliang Cheng1, and Cuiping Ren1
1Department of MRI, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China, 3Department of Radiology, Tianjin Huanhu Hospital, Tianjin, China, 4MR Research China, GE Healthcare, Beijing, China
1Department of MRI, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China, 3Department of Radiology, Tianjin Huanhu Hospital, Tianjin, China, 4MR Research China, GE Healthcare, Beijing, China
Synopsis
Keywords: Stroke, Brain
We aimed to investigate the static and dynamic characteristics of brain activity after stroke by using functional magnetic resonance imaging (fMRI). Seventy-three patients with pontine ischemic stroke and 50 healthy controls (HCs) were recruited and received a resting-state fMRI scanning. Our result shows that there are significantly altered sALFF/dALFF and sReHo/dReHo values in the brain regions of patients. And the alterations of dynamic brain activity in the basal ganglia and cerebellum were correlated with the degree of cognitive impairment. Static and dynamic biomarkers can be combined together to provide more comprehensive information on potential therapeutic targets.Introduction
Pontine infarction (PI) is the most common type of stroke in the posterior circulation territory, accounting for approximately 7% of all ischemic strokes[1]. Clinical symptoms caused by solitary pontine infarction include motor dysfunction[2] and cognitive impairment[3]. However, the underlying mechanism is presently unknown. Resting-state functional magnetic resonance imaging (rs-fMRI) is a promising tool for exploring the functional alterations in the human brain by detecting spontaneous brain activity through low-frequency fluctuations in blood oxygen level-dependent (BOLD) signals. The amplitude of low-frequency fluctuations (ALFF) and regional homogeneity (ReHo) are two of the primary methods of rs-fMRI. In addition, dynamic measurements have the ability to capture recurring brain activity[4], such as dALFF and dReHo. Dynamic research may supplement the deficiencies in static alterations, and their combination may offer a more comprehensive explain for neuropathological changes in chronic PI. However, most studies have used only one or two static or dynamic rs-fMRI parameters to evaluate local or overall functional changes. In this study, we integrated the observation of static and dynamic alterations in the resting-state local metrics including static ALFF (sALFF), static ReHo (sReHo), dynamic ALFF (dALFF), and dynamic ReHo (dReHo) to explore changes in anomalous spontaneous brain activity in the pontine infarction. The analysis of receiver operating characteristic (ROC) was conducted to assess the potential clinical significance of the above methods.Methods
Seventy-three right-handed patients with unilateral pontine infarction consisting of 42 patients with left pontine ischemic (LPI group) and 31 patients with right pontine ischemic (RPI group) were recruited, as well as 50 right-handed healthy controls (HCs) with closely matched age, gender, and education to the patients. All rs-fMRI and T1-weighted images were obtained on a 3.0-Tesla MR imaging system (Discovery MR 750, GE Medical Systems, Waukesha, WI, United States). Parameters for rs-fMRI sequence were as the following : repetition time (TR) = 2,000 ms, echo time (TE) =41 ms, FOV = 220 × 220 mm, flip angle (FA) = 90◦, matrix = 64×64, thickness = 4.0 mm, number of slices = 32, 0.5 mm gap, 190 time points. The 3D T1-weighted images were acquired with a brain volume (BRAVO) sequence, and imaging parameters were as follows: TR/TE = 8.2 ms/3.2 ms; 188 slices; thickness = 1.0 mm; no gap; FA = 12◦; acquisition matrix = 256 × 256; FOV = 256 × 256 mm. The inclusion criteria were patients with first-onset ischemic stroke and chronic-stage pontine infarction. Ray Auditory Verbal Learning Test (RAVLT) and the response accuracy (ACC) of the Spatial 1-back task for each subject was calculated.Result
As is shown in Figure1, compared with the HCs group, the LPI group showed significantly increased sALFF in the left inferior temporal gyrus (ITG_L), left caudate nucleus (CN_L), and right caudate nucleus (CN_R). The RPI group showed increased sALFF in the left caudate nucleus (CN_L) and right caudate nucleus (CN_R), and decreased sALFF in the right cuneus (CUN_R) and left superior occipital gyrus (SOG_L). For dALFF, the LPI group showed significantly increased dALFF in the left inferior temporal gyrus (ITG_L), while the RPI group showed increased dALFF in the left globus pallidus (GP_L), left putamen (PUT_L), right caudate nucleus (CN_R), and left inferior temporal gyrus (ITG_L), and decreased dALFF in the right cuneus (CUN_R), left superior occipital gyrus (SOG_L), and right middle temporal gyrus (MTG_R). No significant differences in sReHo were observed between NC and LPI groups. The RPI group showed significantly decreased sReHo in the right cuneus (CUN_R) and left superior occipital gyrus (SOG_L). For dReHo, the LPI group showed significantly increased dReHo in the left middle temporal gyrus (MTG_L) and decreased dReHo in the right cerebellar lobule VIII (CBE lobule VIII_R). And the RPI group showed significantly decreased dReHo in left middle cingulate cortex (MCC_L) (Figure2). In figure 3, correlation analysis showed that the dALFF in the GP_L and PUT_L in RPI group was negatively correlated with RAVLT scores (p=0.004, r=-0.532) and with Spatial 1-back ACC (p=0.005, r=0.-508). The altered dReHo in the CBE lobule VIII_R was positively correlated with Spatial 1-back ACC (p=0.008, r=0.404). The ROC curves of sALFF/dALFF and sReHo/dReHo were presented in Figure 4 and 5, indicating that sALFF/dALFF and sReHo/dReHo have comparable performance in distinguishing PI patients from the HCs.Discussion and conclusion
In our study, PI patients showed abnormal brain activity in the basal ganglia, visual cortices and cerebellum, which may reflect impaired visual and memory function. The alterations of dynamic brain activity in the basal ganglia and cerebellum were correlated with the degree of cognitive impairment. Furthermore, ROC analysis demonstrated that sALFF/dALFF and sReHo/dReHo had comparable capacity to differentiate PI patients from HCs. Static and dynamic findings can be combined together to provide more comprehensive information for potential therapeutic targets.Acknowledgements
We are deeply grateful to our participants and their families for their generous support for our study.References
1.Huang J, Qiu Z, Zhou P, et al. Topographic location of unisolated pontine infarction. BMC Neurol. 2019 Aug 5;19(1):186.
2. Duncan PW, Goldstein LB, Matchar D, et al. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992 Aug;23(8):1084-9.
3.Obayashi S. Frontal dynamic activity as a predictor of cognitive dysfunction after pontine ischemia. NeuroRehabilitation. 2019;44(2):251-261.
4.Xie H, Calhoun VD, Gonzalez-Castillo J, et al. Whole-brain connectivity dynamics reflect both task-specific and individual-specific modulation: A multitask study. Neuroimage. 2018 Oct 15;180(Pt B):495-504.
Figures
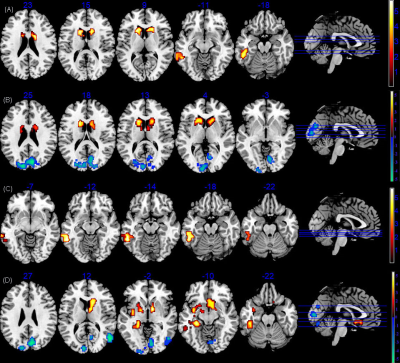
FIGURE 1 The sALFF/dALFF differences between PI and healthy controls. (A)Brain regions with significant sALFF alterations in LPI group. (B) Brain regions with significant sALFF alterations in RPI group. (C)Brain regions with significant dALFF alterations in LPI group. (D)Brain regions with significant dALFF alterations in RPI group. LPI: left pontine infarction; RPI: right pontine infarction.
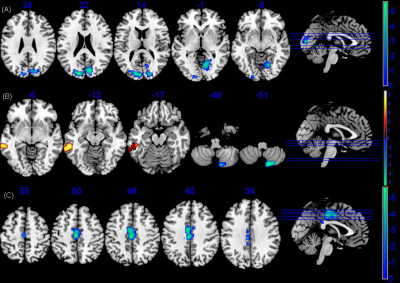
FIGURE 2 The sReHo/dReHo differences between PI and healthy controls. (A)Voxel-base analysis showed brain regions with significant sReHo alterations of RPI group. (B)Voxel-base analysis showed brain regions with significant dReHo alterations of LPI group. (C)Voxel-base analysis showed brain regions with significant dReHo alterations of RPI group. LPI: left pontine infarction; RPI: right pontine infarction.
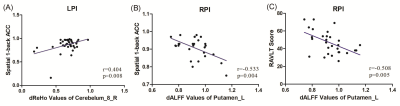
FIGURE 3 Correlations between dALFF/ dReHo values
and behavioral scores. (A) The patients with LPI showed a significantly positive correlation between dReHo values and spatial 1-back ACC
scores. (B) The
patients with RPI showed a significantly negative correlation between dALFF
values and spatial 1-back ACC
scores. (C) The patients with RPI showed a
significantly negative correlation between dALFF values and RAVLT scores. LPI:
left pontine infarction; RPI: right pontine infarction.
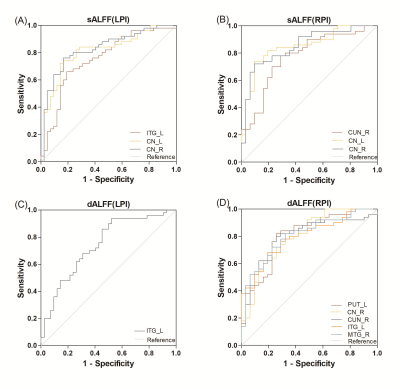
FIGURE 4 ROC analyses of sALFF and dALFF for distinguishing
LPI (A, C) and RPI (B,D) patients from the health controls with (A) sALFF of
ITG_L, CN_L, CN_R (AUC=0.753, 0.810, 0.828); (B) sALFF of CUN_R, CN_L, CN_R(AUC=0.774,
0.849, 0.850); (C) dALFF of ITG_L (AUC=0.744); (D) dALFF of PUT_L, CN_R, CUN_R,
ITG_L, MTG_R (AUC=0.805, 0.799, 0.795, 0.796, 0.804); sALFF and dALFF, static
and dynamic amplitude of low-frequency fluctuations; LPI and RPI, left and right
pontine infarction.
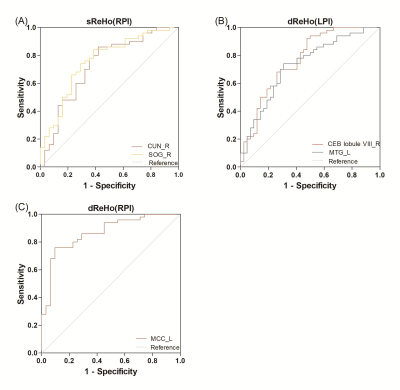
FIGURE 5 ROC analyses of sReHo and dReHo for distinguishing
patients from the health controls. (A) sReHo values of CUN_R (AUC = 0.728), SOG_R(AUC=0.757)
distinguishing RPI patients; (B)dReHo values of CEB lobule VIII_R(AUC=0.767), MTG_L
(AUC=0.731) distinguishing LPI patients; (C) dReHo values of MCC_L (AUC=0.867)
distinguishing RPI patients; sReHo and dReHo, static and dynamic regional
homogeneity; LPI and RPI, left and right pontine infarction.
DOI: https://doi.org/10.58530/2023/1948