1946
Comparison of DWI-ASL Mismatch and Collaterals for Predicting Clinical Outcome in Patients with Subacute Ischemic Stroke1Neusoft Medical Systems Co., Ltd, Shanghai, China, 2Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 3Neusoft Medical Systems Co., Ltd, Shenyang, China
Synopsis
Keywords: Stroke, Stroke, DWI-ASL Mismatch, Collaterals, Subacute troke, Prognosis
As a contrast agent-free MR imaging, ASL will not cause contrast agent-associated nephrotoxicity and allergic reaction. However, low image resolution makes it time-consuming and difficult for clinicians to quantify hypoperfusion volumes using ASL. We developed an automatic and robust algorithm to quantify core-penumbra mismatch based on DWI-ASL. Furthermore, models were built with clinical and/or imaging features and their performances in predicting prognosis of subacute stroke patients were evaluated. When combining infarct location and collaterals with NIHSS, but not mismatch ratio, the model can predict prognosis of subacute stroke patients the best. Therefore, this may add benefits to delayed intervention.
Introduction
Collateral grading for cerebral angiography imaging and ischemic tissue assessment are two critical approaches to quantify salvageable tissue 1 using perfusion imaging techniques that rely on contrast agent, like CTP and DSC-MRI. As a contrast agent-free perfusion technique, the feasibility of ASL to measure core-penumbra mismatch has been demonstrated 2. However, low image resolution makes it time-consuming and difficult for clinicians to quantify hypoperfusion areas using ASL 3. Therefore, we aim to develop an automated quantitative method that quantifies hypoperfused tissue on ASL image and evaluate its performance in predicting prognosis using mismatch ratio and/or collateral score in patients beyond 24 hours from strokeMethods
Subjects: Eighty-six patients (55 ± 10 years; 22F) beyond 24 hours from stroke ictus were retrospectively recruited. Modified Ranking Scale (mRS) score at 90-day follow-up was documented, of which any mRS < 3 was considered as good.Imaging protocol: All MR examinations were performed with 3.0-T scanning (Discovery 750; GE Healthcare, Milwaukee, WI, USA). The imaging parameters were as follows: (1) ASL: TR/TE/PLD/LD=4787/14.63/1525/1500ms, FOV=100×100 mm2, voxel size=1.875×1.87×4.0mm3, slice number=34, matrix=128×128, scan time=4’50’’; (2) DWI: b-value 0 and 1000 s/mm2, TR/TE=3000/65.5 ms, matrix=256×256 , FOV=100×100mm2, voxel size=0.94×0.94×5.0mm3, slice number=20; (3) MRA: TR/TE=21/2.5ms, slice number=152, matrix=512×512, FOV=88×88mm2, voxel size=0.43×0.43×1.2 mm3. The CBF and ADC maps were computed from ASL and DWI images.
Manual quantification: All images were analyzed by a neuroradiologist with 5 years of experience and rectified by another neuroradiologist with 7 years of experience. Infarct core on DWI and hypoperfusion tissue on ASL were semi-automatically delineated using ITK-SNAP (http://www.itksnap.org) by applying thresholds of 620 × 10-6 mm2/s on ADC maps and 5-30 mL/100g/min on CBF maps, respectively 4,5. The collaterals filling on MRA were scored from 0 to 3 according to the grading system proposed by Tan et al 6.
Automatic quantification: (1) The DWI image was used as a reference, to which ASL images were registered; (2) Skulls were removed; (3) WM, GM and CSF were segmented from aligned DWI image; (4) Inhomogeneity bias was corrected and DWI image was normalized to Montreal Neurological Institute coordinates; (5) The partial volume of GM in each voxel of other sequences were calculated using DWI segmentation in step3 and the partial volume was estimated at 50% 7; (6) Infarct tissue was identified and quantified with ADC < 620 × 10-6 mm2/s; (7)The CBF map was quantified according to a previous study 8; (8) After identifying the diseased side, penumbra region was identified and quantified with relative CBF less than 40% contralateral side 9. Step 2-5 were executed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). The mismatch ratio was calculated according to Volumehypoperfusion/Volumeinfarct.
Models to predict prognosis: A total of 5 models were built as follows: (1) Model 1 was built by National Institute of Health stroke scale (NIHSS) which was key to diagnosis and treatment of stroke 10; (2) Model 2: NIHSS + infarct location, with infarct location categorized according to the responsible arteries and likely altering post-stroke recovery potential 11; (3) Model 3/4/5: Model2 combining with core-penumbra mismatch or/and collaterals.
Statistical analysis: SPSS (SPSS Inc., Chicago, USA) and MedCalc (MedCalc, Ostend, Belgium) were used to conduct the statistical analysis. The inter-rater reproducibility for infarct quantification on manual and automatic annotation was evaluated using ICC and Bland-Altman plot. Further, ROC curve was used to assess the ability of mismatch ratio and collateral score to predict good prognosis, with AUC, sensitivity and specificity calculated as well.
Results
The Bland-Altman plots reveals that the mean volumetric difference between the infarct/hypoperfusion volume quantified by manual and automatic way was -1.8/5.0ml (Figures 1&2). No significant differences were observed (P=0.48/0.18).Excellent agreement between manual and automatic quantification of infarct/hypoperfusion was observed (ICCinfarct=95.9%, 95% CI: 0.94, 0.97; ICChypoperfusion=92.4%, 95% CI: 0.88, 0.95)
Figure 3 demonstrates that all models can predict good prognosis accurately (AUC=0.749-0.833). Specifically, the model combining NIHSS, infarct location and collaterals achieves the best performance.
Discussion
The good agreement between this proposed algorithm and manual results demonstrates the capability of our method in quantifying infarct and hypoperfusion volumes. Meanwhile, the increased AUC achieved with model 2 than model 1 suggests that infarct location is a vital factor for predicting prognosis 11. When combining mismatch ratio and/or collaterals, model 4 instead of 5 achieves the best performance, indicating that collaterals can predict good prognosis of subacute stroke patients 12. To our knowledge, this is the first study to compare the ability of core-penumbra mismatch and collaterals in predicting the prognosis of patients with subacute ischemic stroke. Furthermore, our results indicate the potential of collaterals, instead of core-penumbra mismatch, might open the window of intervention beyond 24 hours from last known well.Acknowledgements
No acknowledgement found.References
1. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11-21.
2. Lyu J, Duan Q, Xiao S, et al. Arterial Spin Labeling-Based MRI Estimation of Penumbral Tissue in Acute Ischemic Stroke [published online ahead of print, 2022 Jul 18]. J Magn Reson Imaging. 2022;10.1002/jmri.28364.
3. Liu J, Lin C, Minuti A, Lipton M. Arterial spin labeling compared to dynamic susceptibility contrast MR perfusion imaging for assessment of ischemic penumbra: A systematic review. J Neuroimaging. 2021;31(6):1067-1076.
4. Wang DJ, Alger JR, Qiao JX, et al. The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: comparison with dynamic susceptibility contrast-enhanced MRI. Stroke. 2012;43(4):1018-1024.
5. Purushotham A, Campbell BC, Straka M, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke. 2015;10(3):348-353.
6. Tan BY, Wan-Yee K, Paliwal P, et al. Good Intracranial Collaterals Trump Poor ASPECTS (Alberta Stroke Program Early CT Score) for Intravenous Thrombolysis in Anterior Circulation Acute Ischemic Stroke. Stroke. 2016;47(9):2292-2298.
7. Raoult H, Petr J, Bannier E, et al. Arterial spin labeling for motor activation mapping at 3T with a 32-channel coil: reproducibility and spatial accuracy in comparison with BOLD fMRI. Neuroimage. 2011;58(1):157-167.
8. Wang R, Yu S, Alger JR, et al. Multi-delay arterial spin labeling perfusion MRI in moyamoya disease--comparison with CT perfusion imaging. Eur Radiol. 2014;24(5):1135-1144.
9. Bivard A, Krishnamurthy V, Stanwell P, et al. Arterial spin labeling versus bolus-tracking perfusion in hyperacute stroke. Stroke. 2014;45(1):127-133.
10. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
11. Etherton MR, Rost NS, Wu O. Infarct topography and functional outcomes. J Cereb Blood Flow Metab. 2018;38(9):1517-1532.
12. Messina SA. Collaterals Will Be Key to Opening the Window of Intervention beyond 24 Hours. Radiology. 2022;302(2):408-409.
Figures

Figure 1. The Bland-Altman plot for infarct. The solid line indicates the mean volumetric difference between infarct volumes quantified by manual and automatic way is -1.8ml. The dashed lines represent the 95% confidence interval. No significant difference is observed (P=0.48).
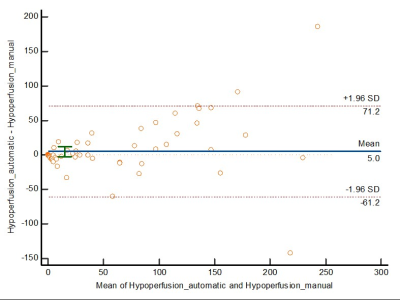
Figure 2. The Bland-Altman plot for hypoperfusion volumes. The mean volumetric difference between the hypoperfusion volume quantified by manual and automatic way is 5.0ml. The dashed lines represent the 95% confidence interval. No significant difference is observed (P=0.18).
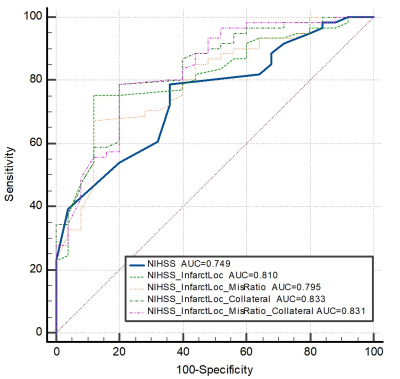
Figure 3. The diagnostic performance of National Institute of Health stroke scale (NIHSS), infarct location, collaterals and mismatch ratio to predict prognosis. The model combining NIHSS, infarct location and collaterals performs best in predicting prognosis (AUC=0.833). It is worth noting that the accuracy has been greatly improved after adding image indicators such as infarct location. While core-penumbra mismatch has a slightly negative effect on the prediction model (P <0.001).