1940
Evaluation of Brain Iron Deposition in Different Cerebral Artery of Acute Ischemic Stroke by using Quantitative Susceptibility Mapping1Department of Radiology, Zigong First People's Hospital, Zigong, China, 2North Sichuan Medical College, Nanchong, China, 3Sichuan Vocational College of Health and Rehabilitation, Zigong, China, 4MR Scientific Marketing, SIEMENS Healthineers Ltd., Shanghai, China
Synopsis
Keywords: Stroke, Blood vessels, Iron Deposition
In this study, QSM was used to investigate susceptibility differences among infarct region, non- infarct regions of responsible artery and non-responsible artery in acute ischemic stroke (AIS) patients. The results showed that only susceptibility of the infarct region was significantly higher than that of healthy controls. In AIS patients, the susceptibility of infarct region were significantly higher than those of normal cerebral artery regions and non-infarction regions of responsible artery. These results suggest that abnormal iron deposition exists in the infarct region and may not affect other non-infarct regions.Introduction
Acute ischemic stroke (AIS), a common type of stroke, is one of the leading causes of death and disability among worldwide1. Although studies have shown that middle cerebral artery stenosis is accompanied by abnormal iron deposition in gray matter nuclei2, there are few studies focus on the iron deposition differences in various cerebral artery regions in AIS patients. In this study, quantitative susceptibility mapping (QSM) was used to investigate the difference of iron deposition between infarct region and normal cerebral artery regions in AIS patients.Methods
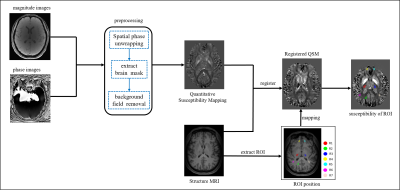
MR imaging: Thirty patients with acute ischemic stroke (20 males and 10 females; age: 46-91 years; mean age: 69.367 years; standard deviation (SD): 10.515) and thirty healthy controls (13 males and 17 females; age: 45-78 years; mean: 60.129, SD: 8.672) in the study. MRI data were acquired using 3T MR scanner (MAGNETOM VIDA, Siemens Healthineers, Erlangen, Germany), with a 64-channel head coil. Quantitative sensitivity mapping were acquired using multi-echo GRE sequence, and the parameters were as following: repetition time (TR) =55.0 ms, echo time (TE) = 6.15 ms, flip angle = 15°, field of view (FOV) = 220 × 220 mm2, thickness = 2 mm, number of slices = 72, voxel size = 0.9 × 0.9 × 2 mm3, SMS factor = 3, parallel imaging with acceleration factor = 2 (total PAT = 6) , 8 echoes, total scan time is 8min 7sec. High-resolution T1-weighted anatomical 3D scans using MPRAGE sequence with the following parameters: TR = 2300 ms, TE = 2.45 ms, flip angle =9°, FOV =256 × 256 mm2, thickness = 0.9 mm, number of slices = 176, voxel size = 1 × 1 × 1 mm3, total scan time is 4min 8sec.Image Processing: The QSM data were post-processed using MEDI toolbox (Morphology Enabled Dipole Inversion) based on Matlab 2018a software platform (Mathworks, Natick, MA, USA). The QSM images were registered to the 3D T1-weighted imaging using 3D Slicer software. The infarct region of responsible artery (R1), the non-infarct region of responsible artery (R2), the contralateral symmetrical site of the lesion (R3), and the non-responsible cerebral arteries regions (R4, R5, R6, R7) of the AIS patients were selected as the regions of interest (ROI). For the healthy control group, the cerebral arteries region corresponding to the patient was selected as the ROI. ITK-SNAP was used to identify and delineate ROIs in the 3D T1 image, and then copied to the QSM image. The process is shown schematically in Figure 1.
Statistical Analysis: Two-sample T-test was used to compare the differences in corresponding ROI susceptibility between AIS patients and healthy controls, and paired T-test was used to compare the differences in susceptibility between infarct region and non-infarct regions in AIS patients.
Result
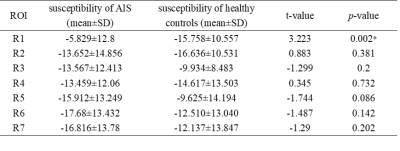
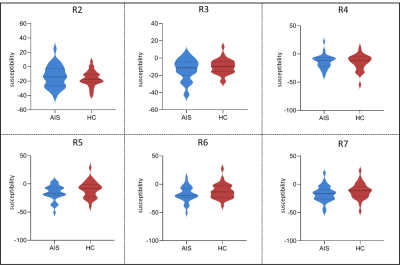
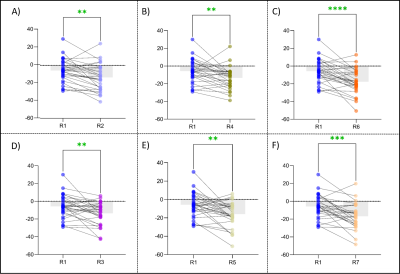
Using Two-sample T-test, the results showed that the susceptibility of infarct region in AIS patients was significantly higher than that in healthy controls (p = 0.002). There was no significant difference in non-infarct regions between AIS patients and healthy controls, although the mean susceptibility of R3, R5, R6, and R7 was higher in healthy controls than in AIS patients (all p > 0.05) (Table 1, Figure 2). Using paired T-test, the result showed that the susceptibility of R1 in AIS patients was significantly higher than that of R2 and R3 (p < 0.05). Compared with the non-responsible cerebral artery regions, the susceptibility of the infarct region was significantly increased (p < 0.05) (Figure 3).Discussion
This study found that the brain iron deposition in the infarct region was significantly higher than that in the healthy control group, which may due to the release of iron ions from the cells in a potentially toxic form in the ischemic and anoxic environment, leading to abnormal iron deposition in the infarction region3, 4. The susceptibility of AIS patients in infarct region is significantly higher than that in non-infarct regions of responsible artery, which may be related to the opening of collateral circulation of cerebral vessels5. In AIS patients, the susceptibility of the infarct region was significantly higher than that of the contralateral symmetrical site of the lesion and the non-responsible cerebral arteries region, which indicates that the local infarct region of the AIS responsible vessel does not affect the iron level in the contralateral region and other normal cerebral arteries region. Therefore, QSM may be a useful and promising tool to assess changes in brain iron level after ischemic stroke.Conclusion
Abnormal iron deposition detected by QSM in the infarct region of AIS patients may not affect the iron level in the non-infarct region of responsible artery and normal cerebral arteries, which may provide imaging reference for clinical diagnosis.Acknowledgements
We are particularly grateful to all those who helped us with the article.References
1. Feigin, VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet Neurology, 2021. 20(10): p. 795-820.
2. Mao, H, Dou W, Chen K, et al. Evaluating iron deposition in gray matter nuclei of patients with unilateral middle cerebral artery stenosis using quantitative susceptibility mapping. NeuroImage: Clinical, 2022. 34: p. 103021.
3. Chiou, B, Neal EH, Bowman AB, et al. Endothelial cells are critical regulators of iron transport in a model of the human blood–brain barrier. Journal of Cerebral Blood Flow & Metabolism, 2019. 39(11): p. 2117-2131.
4. Campos-Escamilla, C. The role of transferrins and iron-related proteins in brain iron transport: applications to neurological diseases. Advances in protein chemistry and structural biology, 2021. 123: p. 133-162.
5. Sallustio, F, Motta C, Pizzuto S, et al. CT angiography-based collateral flow and time to reperfusion are strong predictors of outcome in endovascular treatment of patients with stroke. Journal of neurointerventional surgery, 2017. 9(10): p. 940-943.
Figures