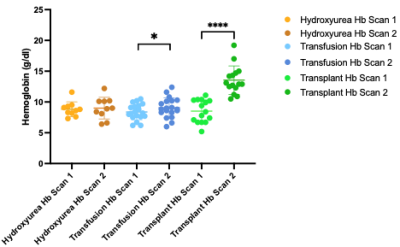1938
Varied hemodynamic and metabolic responses of disease modifying and curative therapies in adults with sickle cell disease1Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Radiology, Vanderbilt University Medical Center, Nashville, TN, United States, 3Pediatric Endocrinology and Diabetes, Vanderbilt University Medical Center, Nashville, TN, United States, 4Pediatrics, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Keywords: Stroke, Stroke, metabolism
Two common treatment paradigms for sickle cell disease (SCD) include oral medication hydroxyurea and blood transfusions. Rarely, a curative hematopoietic stem cell transplant (HSCT) is performed. We utilized non-invasive MRI to evaluate how potential biomarkers of infarct risk, cerebral blood flow (CBF) and oxygen extraction fraction (OEF), change in these treatment paradigms (cumulative n=39). Hydroxyurea treatment did not have significant changes to hemoglobin, CBF, or OEF between two scans. Hemoglobin increases by approximately 8.73 and 64.11% for adults with SCD on transfusion and transplant treatments, respectively. These changes most prominently parallel a reduction in CBF of 20.46 and 31.67%, respectively.Introduction
The overall goal of this work is to use multi-modal functional neuroimaging to evaluate cerebral hemodynamic changes in sickle cell disease (SCD) patients across a variety of conservative (hydroxyurea) and more aggressive (red cell exchange transfusion and hematopoietic stem cell transplant, HSCT) treatments. SCD is a genetically-inherited blood disorder that results in the production of hemoglobin-S (HbS); the presence of erythrocytes with HbS results in hemolysis and associated anemia, and an elevated risk for cerebrovasculopathy, silent cerebral infarcts, and stroke. Standard-of-care in most SCD patients without cerebral infarcts is oral hydroxyurea, which is myelosuppressive and increases fetal hemoglobin (HbF). Patients may also receive more aggressive monthly blood transfusions which increase total Hb by 1-2 g/dl on average, or, in high-risk patients, HSCT, which is a curative therapy when engraftment is successful. In adults with SCD, there is no current standard for stratifying more aggressive therapeutic options such as transfusion and transplant for prevention of stroke or other serious disease complications. Elevated brain oxygen extraction fraction (OEF)1,2 and cerebral blood flow (CBF)3–7 have previously been identified as possible biomarkers for treatment selection. If so, these parameters should respond to effective treatments which reduce cerebral infarct burden. The purpose of this study was to utilize noninvasive pseudo-continuous arterial spin labeling (pCASL) and T2-relaxation-under-spin-tagging (TRUST) MRI methods to quantify how CBF and OEF change in response to disease-modifying and curative SCD treatments.Methods
Study participants. Adult participants (n=39) with SCD (hemoglobin phenotype: HbSS or HbSC), provided informed consent for this prospective study, and were scanned at 3T (Philips) at two time points. All treatments were clinically-indicated. 10 participants were receiving hydroxyurea treatment, 19 participants were scanned pre- and post-blood transfusion, and 10 participants were scanned pre- and post-HSCT, with the second scan occurring approximately one year following transplant.Experiment. Total Hb and Hb-S fraction (HbS%) were measured via venipuncture and arterial oxygen saturation (Ya) via pulse oximetry at the time of imaging. 3D T1-weighted MPRAGE (TR/TE=8.2/3.7ms; spatial resolution=1.0x1.0x1.0mm3), 2D T2-weighted FLAIR (axial and coronal; TR/TE=11,000/120ms; spatial resolution=1.0 x 1.1 x 4.0 mm3), 2D pseudo-continuous ASL (label delay=1900 ms; spatial resolution=3x3x7 mm3), and TRUST (TR/TE=1978/3.6 ms; spatial resolution=3.4x3.4x5 mm3; τCPMG=10ms; eTE=0, 40, 80, and 160ms) were performed for anatomical, CBF, and OEF assessments.
Analyses. Total gray matter CBF was quantified using procedures reported in the literature for patients with SCD, accounting for individual effects of Hb on blood T14. Global OEF was calculated from TRUST upon pair-wise subtraction of data and quantification of venous blood water R2 and subsequently venous oxygen saturation using procedures outlined in the literature for patients with SCD8. OEF was calculated as the fractional difference in arterial oxygen saturation measured from pulse oximetry and venous oxygenation measured from TRUST. Hemodynamic and metabolic measures were pair-wise compared within each cohort using a paired Student’s t-test (significance: two-sided p<0.05); OEF and CBF changes vs. fractional changes in Hb are presented as scatter plots with associated slopes of best-fit lines and Pearson’s correlation coefficients.
Results
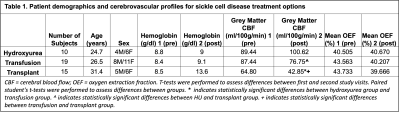
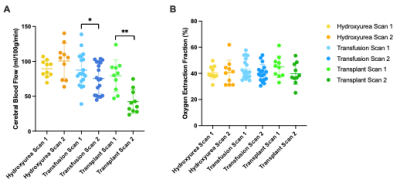
Table 1 summarizes the demographics for each cohort. In adults with SCD stable on hydroxyurea treatment, Hb did not change (p=0.63) between time 1 (Hb=8.8±1.2 g/dl) and time 2 (Hb±9.00+/-1.76 g/dl) and this was associated with no significant change between time points for CBF (p=0.24) or OEF (p = 0.77) (Fig. 1). In patients receiving blood transfusion treatment, Hb increased from 8.38±1.28 g/dl to 9.06±1.62 g/dl (p = 0.003) pre- vs. post-transfusion (p = 0.003), which paralleled a significant decrease in CBF of 11.9 ml/100g/min (p = 0.019) after transfusion (Fig. 2). OEF did not change significantly (pre: 43.56%; post: 40.21%; p=0.37). In transplant patients, results show significant increases in hemoglobin from 8.51±1.84 g/dl to 13.60±2.31g/dl (p = 0.003) pre- vs. post-transplant, which paralleled significant decreases in CBF from 79.4±23.01 ml/100g/min to 42.4±16.8 ml/100g/min (p = 0.005). OEF trended (p=0.16) lower post-transplant with an absolute reduction of 12.52%, but this change did not meet stated significance criteria. Fig. 3 shows the change in CBF and OEF for treatment-induced changes in Hb across all participants: across all participants, CBF decreased by 9.96 ml/100g/min per g/dl increase in hemoglobin, whereas OEF decreased by 1.56 per g/dl increase in Hb.Discussion
Increases in Hb were larger from pre- to post-HSCT than pre- to post-transfusion, which translated to a larger mean decrease in CBF post-transplant. Disease modifying therapies improve CBF and curative HSCT has the greatest impact on CBF. Findings also support that CBF and OEF are more dynamic following transfusion and transplant therapies than in patients on stable doses of conservative hydroxyurea, highlighting the potential relevance of these methods for evaluating emerging pharmacological or genetic therapies. Finally, the demonstration that CBF and OEF responds to even subtle changes in Hb provides a framework for motivating these measures as endpoints in emerging SCD therapy trials.Conclusion
Hb increases by approximately 8.73 and 64.11% for adults with SCD receiving transfusion and transplant treatments, respectively. These changes most prominently parallel a reduction in CBF of 20.46 and 31.67%, respectively.Acknowledgements
No acknowledgement found.References
1. Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain. 2016;139(3):738-750. doi:10.1093/brain/awv3972.
2. Ford AL, Ragan DK, Fellah S, et al. Silent infarcts in sickle cell disease occur in the border zone region and are associated with low cerebral blood flow. Blood. 2018;132(16):1714-1723. doi:10.1182/blood-2018-04-8412473.
3. Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40(3):383-396. doi:10.1002/mrm.19104003084.
4. Juttukonda MR, Jordan LC, Gindville MC, et al. Labeling Considerations in Adults With Sickle Cell Anemia. 2018;30(2):1-21. doi:10.1002/nbm.3681.Cerebral5.
5. Watchmaker JM, Juttukonda MR, Davis LT, et al. Hemodynamic mechanisms underlying elevated oxygen extraction fraction (OEF) in moyamoya and sickle cell anemia patients. J Cereb Blood Flow Metab. 2018;38(9):1618-1630. doi:10.1177/0271678X166825096.
6. Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60(2):357-363. doi:10.1002/mrm.216277.
7. Bush A, Borzage M, Detterich J, et al. Empirical model of human blood transverse relaxation at 3 T improves MRI T 2 oximetry. Magn Reson Med. 2017;77(6):2364-2371. doi:10.1002/mrm.263118.
8. Juttukonda MR, Donahue MJ, Waddle SL, et al. Reduced oxygen extraction efficiency in sickle cell anemia patients with evidence of cerebral capillary shunting. J Cereb Blood Flow Metab. 2021;41(3):546-560. doi:10.1177/0271678X20913123
Figures