1936
Regional age-related changes of neuromelanin and iron in the substantia nigra based on neuromelanin accumulation and iron deposition1Shandong Provincial Hospital, Shandong University, Jinan, China, 2Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China, 3Department of Radiology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China, 4Philips Healthcare, Shanghai, China
Synopsis
Keywords: Neurodegeneration, Aging
The study investigated age-related neuromelanin signal variation and iron content changes in the subregions of substantia nigra simultaneously by magnetization transfer contrast neuromelanin-sensitive multi-echo fast field echo sequence in a normal population. We manually delineated neuromelanin, iron deposition regions and the overlap regions. The correlation analysis revealed contrast ratio increased over age, while volume showed an age-related decline in the overlap region which were similar to those in the neuromelanin region. No significant correlations were found between susceptibility and age in any subregion. This study may provide more insight for the future degenerative elucidations of substantia nigra.Introduction
The substantia nigra (SN) in the midbrain, which is rich in dopamine neurons, plays an important role in regulating movement control, cognitive development, and emotional processes1,2. Neuromelanin (NM) is biosynthesized from dopamine in the substantia nigra pars compacta (SNpc) 3. The loose substantia nigra pars reticulata (SNr) contains a higher iron content than the SNpc in normal subjects 4, 5. There is no sharp demarcation between the areas of the SNpc and SNr because neurons in the SNpc form clusters of cells (nigrosomes) that penetrate deep into the SNr 6. Given the inconsistent and crossover spatial distribution of neuromelanin-rich and iron 6–8, there is an overlap region containing both neuromelanin and iron in abundance 5. The purpose of the study is to investigate age-related neuromelanin signal variation and iron content changes in the subregions of substantia nigra using magnetization transfer contrast (MTC) neuromelanin-sensitive multiecho fast field echo (FFE) sequence in a normal population.Methods
In this prospective study, 115 healthy volunteers between 20 and 86 years of age were recruited and scanned using 3.0 T MRI. We manually delineated neuromelanin hyperintensity and iron deposition regions in neuromelanin image and quantitative susceptibility mapping (QSM), respectively. We calculated the overlap region using the two measurements mentioned above. Partial correlation analysis was used to evaluate the correlations between volume, contrast ratio (CR), susceptibility of three subregions of SN and age.Curve estimation models were used to find the best regression model.Results
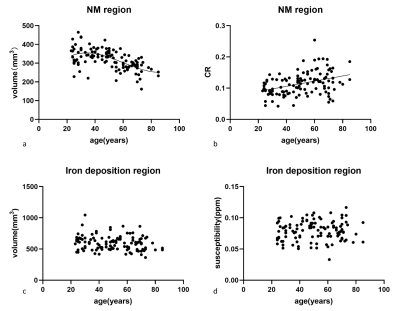
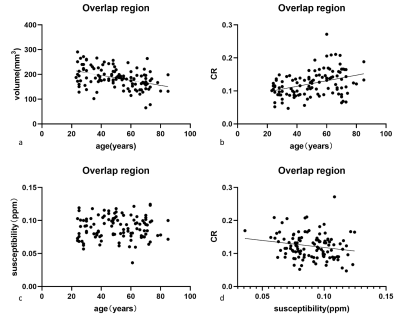
CR increased with age (r=0.379, p<0.001; r=0.371, p<0.001), while volume showed an age-related decline (r=−0.559, p<0.001; r=−0.410, p<0.001) in the NM (Fig.1a and b) and overlap (Fig.2) regions. Cubic polynomial regression analysis found a small increase in NM volume with age until 34, followed by a significant decrease until 80s (R2=0.358, p<0.001). No significant correlations were found between susceptibility and age in any subregion.Discussion
We sought to define the changes in neuromelanin and iron in the overlap region of the substantia nigra with age. Our results indicated that CR increased with age, whereas volume showed an age-related decline in the overlap and NM regions in the normal population. We illustrated an age-independent effect on the volume and CR of the overlap region. This study enriches the age-related literature on neuromelanin in the substantia nigra.In line with the aforementioned studies, the enhancement of neuromelanin in the NM region with age in our study manifested the accumulation of neuromelanin in the dopaminergic neurons.Furthermore, in non-linear regression analysis, there was an increase until 34 years old, followed by a decline in the volume into the 80s. However, the fit analysis and result have not been described in the MRI literature. The more nuanced estimation was consistent with the observation that a significant loss of pigmented neurons occurs in older adults than in younger adults 10. The above results can be supported by the widely recognized neurobiology research that a smaller amount but significant hypertrophy of the pigmented neurons 10 in older controls led to a higher concentration of NM 11.
The changes in volume and CR in the overlapping region were similar to those in the NM region. This can be interpreted as follows: The dopaminergic neurons of group A9 constitute the ventral tier of the nigrosome 5, while the NM is rich in the nigral matrix and nigrosomes.
We did not find a change in volume or susceptibility with age in the iron deposition region. In the literature, findings about the age-related iron change on substantia nigra remain controversial. Consistent with our results, some imaging research reported that the iron content in the substantia nigra was unaltered with age. 9,10
However, some researchers found that susceptibility in substantia nigra increased with age. 12 This inconsistency was potentially due to the differences in the age distribution of the subjects and the sample size. This conflicting evidence indicates that iron accumulation during life span is non-linear or the change during mid-adult phase is subtle.
Conclusion
In conclusion, we investigated temporal changes in neuromelanin and iron content in the subregions of the substantia nigra using the MTC neuromelanin-sensitive multiecho FFE sequence. The results illustrated an age effect in the volume and CR of the overlap region, whereas CR was unaffected by the iron effect. We further found that the volume of the NM region increased until 30s and then decreased until 80s. This study may provide a reference for future neurodegenerative studies of the substantia nigra.Acknowledgements
The authors gratefully thank all volunteers for their participation and support in the study. This work was supported in part by the Natural Science Foundation of Shandong (grant number: ZR2020QH267), the China Postdoctoral Science Foundation (grant number: 2022M711987) and the Taishan Scholars Project (grant number: tsqn201812147).References
1. Nieoullon A (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol 67:53–83. https://doi.org/10.1016/S0301-0082(02)00011-4
2. Liu C, Kaeser PS (2019) Mechanisms and regulation of dopamine release. Curr Opin Neurobiol 57:46–53. https://doi.org/10.1016/j.conb.2019.01.001
3. Fedorow H, Tribl F, Halliday G, et al (2005) Neuromelanin in human dopamine neurons: Comparison with peripheral melanins and relevance to Parkinson’s disease. Prog Neurobiol 75:109–124. https://doi.org/10.1016/j.pneurobio.2005.02.001
4. Chinta SJ, Andersen JK (2005) Dopaminergic neurons. Int J Biochem Cell Biol 37:942–946. https://doi.org/10.1016/j.biocel.2004.09.009
5. Lehéricy S, Bardinet E, Poupon C, et al (2014) 7 tesla magnetic resonance imaging: A closer look at substantia nigra anatomy in Parkinson’s disease: 7T MRI in PD. Mov Disord 29:1574–1581. https://doi.org/10.1002/mds.26043
6. Giovanni G, Di Matteo V, Esposito E (2009) Birth, Life and Death of Dopaminergic Neurons in the Substantia Nigra. Springer Vienna, Vienna
7. Snyder AM, Connor JR (2009) Iron, the substantia nigra and related neurological disorders. Biochim Biophys Acta BBA - Gen Subj 1790:606–614. https://doi.org/10.1016/j.bbagen.2008.08.005
8. Biondetti E, Santin MD, Valabrègue R, et al (2021) The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain 144:3114–3125. https://doi.org/10.1093/brain/awab191
9. Hashido T, Saito S (2016) Quantitative T1, T2, and T2* Mapping and Semi-Quantitative Neuromelanin-Sensitive Magnetic Resonance Imaging of the Human Midbrain. PLOS ONE 11:e0165160. https://doi.org/10.1371/journal.pone.0165160
10. Rudow G, O’Brien R, Savonenko AV, et al (2008) Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol (Berl) 115:461–470. https://doi.org/10.1007/s00401-008-0352-8
11. Zecca L, Gallorini M, Schünemann V, et al (2001) Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes: Nigral iron, neuromelanin and ferritin. J Neurochem 76:1766–1773. https://doi.org/10.1046/j.1471-4159.2001.00186.x
12. Persson N, Wu J, Zhang Q, et al (2015) Age and sex related differences in subcortical brain iron concentrations among healthy adults. NeuroImage 122:385–398. https://doi.org/10.1016/j.neuroimage.2015.07.050
Figures