1934
Combined Microstructural Assessment of Progressive Apraxia of Speech by Diffusion Tensor Imaging-Based Tractography and Multi-Shell NODDI1Division of Neurology, Mayo Clinic at Rochester, Rochester, MN, United States, 2Division of Psychiatry and Psychology, Mayo Clinic at Rochester, Rochester, MN, United States, 3Division of Radiology, Mayo Clinic at Rochester, Rochester, MN, United States
Synopsis
Keywords: Neurodegeneration, Rare disease, Progressive Apraxia of Speech
Progressive apraxia of speech (PAOS) is a tauopathy characterized by difficulties with motor speech programming and planning. Grey and white matter (GM & WM) brain regions were interrogated by diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Twenty-three patients with PAOS and 22 matched controls underwent diffusion MRI. Global WM differences in PAOS were better attained by intracellular volume fraction (ICVF), whereas GM global differences were better attained by mean diffusivity (MD) and isotropic volume fraction (isoVF). DTI and NODDI represent unique aspects of brain tissue microstructure and can be used as PAOS biomarkers.INTRODUCTION:
Progressive apraxia of speech (PAOS) is a tauopathy characterized by difficulties with motor speech programming and planning1. Neurodegeneration in PAOS targets grey and white matter (GM & WM) microstructure that can be assessed using diffusion tensor imaging (DTI). However, muti-shell applications, such as neurite orientation dispersion and density imaging (NODDI) are limited. We hypothesize that the combination of DTI and NODDI can add further insight into microstructural damage in PAOS.METHODS:
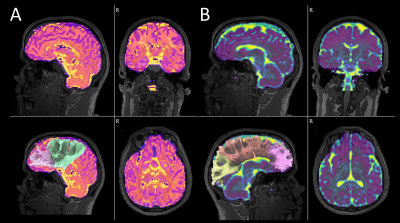
Twenty-three patients with PAOS and 22 age and sex-matched controls were recruited by the Neurodegenerative Research Group (NRG) at Mayo Clinic and underwent diffusion MRI. DTI and whole brain tractography were achieved using DSI studio. NODDI was calculated with MATLAB algorithms2. Outputs were matched using the automated anatomical parcellation atlas (AAL2) in single-subject space (Figure 1). Separately for left and right, fractional anisotropy (FA), mean diffusivity (MD) from DTI, intracellular volume fraction (ICVF), and an isotropic volume fraction (IsoVF) from NODDI, were calculated from frontal, temporal, and parietal GM ROIs. Corpus callosum, frontal aslant tract, arcuate fasciculi, inferior longitudinal fasciculi, and thalamic radiations anterior and corticostriatal WM tracts were analyzed.RESULTS:
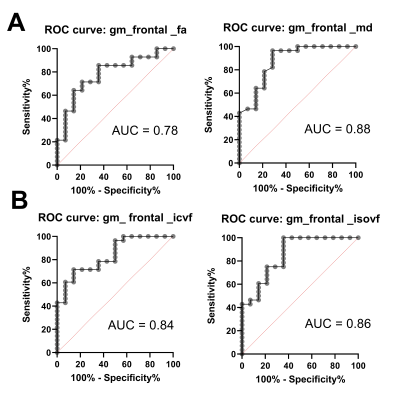
Global WM differences between PAOS and controls were observed in ICVF (p<0.001) and MD (p<0.0053), whereas global GM differences were observed in MD p<0.0010) and IsoVF (p<0.0005). On averaged WM tracts, MD was significantly higher (p<0.0001), ICVF was lower (p<0.003), and IsoVF was higher (p<0.038) in PAOS. Receiver operator characteristic curve (AUROC) results are presented (Figure 2).CONCLUSIONS:
DTI and NODDI represent different aspects of brain tissue microstructure, and both show potential as disease biomarkers in PAOS. Future studies will look at measures relative to symptom severity, to explore NODDI as a biomarker for earlier disease detection.Acknowledgements
This study was funded by NIH grants: R01-DC12519, R01-DC14942References
1. Josephs KA, Duffy JR, Clark HM, Utianski RL, Strand EA, Machulda MM, Botha H, Martin PR, Pham NTT, Stierwalt J, Ali F, Buciuc M, Baker M, Fernandez De Castro CH, Spychalla AJ, Schwarz CG, Reid RI, Senjem ML, Jack CR, Jr., Lowe VJ, Bigio EH, Reichard RR, Polley EJ, Ertekin-Taner N, Rademakers R, DeTure MA, Ross OA, Dickson DW, Whitwell JL. A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nat Commun. 2021;12(1):3452. Epub 2021/06/10. doi: 10.1038/s41467-021-23687-8. PubMed PMID: 34103532; PMCID: PMC8187627.
2. Raghavan S, Przybelski SA, Reid RI, Lesnick TG, Ramanan VK, Botha H, Matchett BJ, Murray ME, Reichard RR, Knopman DS, Graff-Radford J, Jones DT, Lowe VJ, Mielke MM, Machulda MM, Petersen RC, Kantarci K, Whitwell JL, Josephs KA, Jack CR, Vemuri P. White matter damage due to vascular, tau, and TDP-43 pathologies and its relevance to cognition. Acta Neuropathologica Communications. 2022;10(1):16. doi: 10.1186/s40478-022-01319-6.
Figures