1923
Investigation of the biophysical basis of relaxivity contrast imaging as a biomarker for myofiber microstructural changes in an ALS model
Natenael B Semmineh1, Ethan Mathew2, Bruce Damon3, and C Chad Quarles1
1Cancer Systems Imaging, The university of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Barrow Neuroimaging Innovation Center, Barrow Neurological Institute, Phoenix, TX, United States, 3Carle Clinical Imaging Research Program Stephens Family Clinical Research Institute Carle Health, Urbana, IL, United States
1Cancer Systems Imaging, The university of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Barrow Neuroimaging Innovation Center, Barrow Neurological Institute, Phoenix, TX, United States, 3Carle Clinical Imaging Research Program Stephens Family Clinical Research Institute Carle Health, Urbana, IL, United States
Synopsis
Keywords: Neurodegeneration, Microstructure, amyotrophic lateral sclerosis - ALS
Amyotrophic lateral sclerosis (ALS) is a fatal upper and lower motor neuron degradation disease that leads to progressive myofiber abnormalities (e.g., decreased size and distribution). The relaxivity contrast imaging (RCI) parameter, TRATE (tissue transverse relaxivity at tracer equilibrium), has been shown to decrease during ALS induced myofiber degradation. The goal of this study is to expand a validated computational method to investigate the biophysical basis of TRATE in muscles of ALS patients and to optimize RCI acquisition parameters.Introduction
Amyotrophic Lateral Sclerosis (ALS) is characterized by loss of spinal and cortical motor neurons, resulting in progressive muscle atrophy. Recently, we proposed the use of relaxivity contrast imaging (RCI) as a novel approach to evaluate ALS progression and provide faster and improved decision-making during clinical trials [1, 2]. RCI is a susceptibility-based dynamic contrast enhanced approach and the derived biomarker termed the tissue transverse relaxivity at tracer equilibrium (TRATE) was found to be highly sensitive to myofiber size, density, geometry, and heterogeneity [3]. Our goal is to further investigate the biophysical basis of TRATE using an established computational approach in the context of myofiber degeneration.Methods
Computation of MRI signals for 3D tissue structures were carried out using a validated computational strategy termed the finite perturber finite difference method [4,5]. To assure realistic muscle tissue representation, computed RCI data were compared for two input 3D tissue structures obtained from immunofluorescence microscopy and tissue structures modeled as packed elliptical cylinders (Figure 1). The use of elliptical cylinders allows systematic variation that mimic the known histopathologic progression of ALS-induced muscle degeneration. Each myofiber property can be individually adjusted (e.g., diameter, aspect ratio, orientation angle, etc) to systematically characterize their influence on RCI data and capture the heterogeneity observed during disease progression. Additional input parameters including the contrast agent transfer coefficient (Ktrans), water diffusion coefficient and pre-contrast T1 were distinctly chosen to represent values observed in muscle. A multi-echo GRE pulse sequence is modeled to quantify the T2* and T1 changes (ΔR2*(t) and ΔR1(t)) that occur after the injection of a contrast agent (CA). TRATE values are then computed using ΔR2*/Ct for the last few averaged time points. Similar to DCE-MRI, the tissue CA concentration (Ct) is computed using ΔR1/r1, where r1 is the CA’s (known) T1 relaxivity.Results and Discussion
Example 2D section of the 3D muscle tissue structures from histopathologic data at two time points of ALS progression are illustrated in Figure 1 A-B. The corresponding structures constructed using elliptical cylinders are shown in Figure 1 C-D. By matching the mean fiber diameter and volume fraction measured from quantitative histologic analysis, the elliptical cylinders provide similar ΔR2*(t) values to that obtained from histopathologic data for both time points. Figure 2 shows TRATE characterization for four different parameters. Figure 2A shows an increase in TRATE values with increasing fiber diameter. The dependence of TRATE on fiber volume fraction is shown in Figure 2B. Mimicking ALS disease progression can be achieved by inducing loss of myofiber diameter and/or volume fraction. Consistent with in vivo observations [1,2], TRATE values decrease with myofiber degradation in both cases. TRATE values were also found to rely on the mean fiber orientation with respect to the main magnetic field Bo (Figure 2C). Except for the case where the fiber aligns with Bo, a given orientational angle yields similar TRATE values as compared to its complementary angle. This can be explained by the symmetrical field perturbation patterns produced by complementary angles which leads to similar standard deviation of the field perturbation and thereby ΔR2*. Maximum TRATE values are achieved when mean fiber direction is perpendicular to Bo. When the fiber orientation is parallel to Bo the field perturbation outside fibers is zero with only spatially constant field perturbation inside the fibers; hence the relatively small TRATE values for 0o. TRATE and diffusion exhibit an inverse relationship, consistent with diffusion narrowing of field perturbation (Figure 2D).Conclusion
Results of this study further characterize the biophysical features that contribute to TRATE and can aid in the design and interpretation of ongoing and future in vivo preclinical and clinical studies. Currently the computational model is being trained using preclinical data for optimization of sequence parameters to maximize the sensitivity of TRATE to muscle degenerative changes associated with ALS.Acknowledgements
NIH/ NINDS R61NS119642-01References
- Laura C. Bell, Alberto E. Fuentes, Deborah R. Healey, Renee Chao, Nadine Bakkar, Rachael W. Sirianni, David X. Medina, Robert P. Bowser, Shafeeq S. Ladha, Natenael B. Semmineh, Ashley M. Stokes, C. Chad Quarles. Longitudinal evaluation of myofiber microstructural changes in a preclinical ALS model using the transverse relaxivity at tracer equilibrium (TRATE): A preliminary study. Magnetic Resonance Imaging, Volume 85, 2022.
- Ragunathan, S.; Bell, L.C.; Semmineh, N.; Stokes, A.M.; Shefner, J.M.; Bowser, R.; Ladha, S.; Quarles, C.C. Evaluation of Amyotrophic Lateral Sclerosis-Induced Muscle Degeneration Using Magnetic Resonance-Based Relaxivity Contrast Imaging (RCI). Tomography 2021, 7, 169–179. https://doi.org/10.3390/ tomography7020015
- Semmineh, N. B., Xu, J., Skinner, J. T., Xie, J., Li, H., Ayers, G., & Quarles, C. C. (2015). Assessing tumor cytoarchitecture using multiecho DSC- MRI derived measures of the transverse relaxivity at tracer equilibrium (TRATE). Magnetic Resonance in Medicine, 74(3), 772–784.
- Semmineh NB, Stokes AM, Bell LC, Boxerman JL, Quarles CC. A population based digital reference object (DRO) for optimizing dynamic susceptibility contrast (DSC) MRI methods for clinical trials. Tomography n.d.
- Semmineh NB, Xu J, Boxerman JL, Delaney GW, Cleary PW, Gore JC, et al. An efficient computational approach to characterize DSC-MRI signals arising from three-dimensional heterogeneous tissue structures. PLoS One 2014;9. Doi: 10.1371/journal.pone.0084764.
Figures
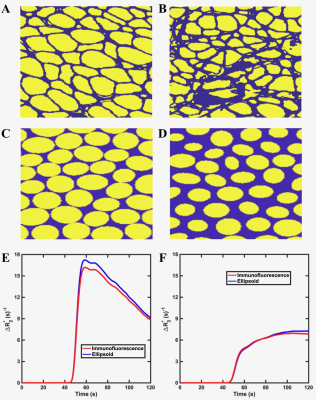
Figure 1: Example 2D section of the 3D muscle tissue structures from histopathologic (A-B) and elliptical cylinders (C-D). (A and C) model early stage fibers and , (B and D) model late stage fibers. For both time points, the histological derived structures and the simulated tissue give similar ΔR2* time curves (E-F).
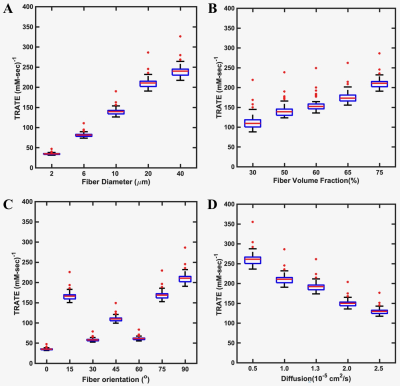
Figure 2: The dependence of TRATE on fiber diameter (A), fiber volume fraction (B), fiber orientation (C) and mean diffusivity (D).
DOI: https://doi.org/10.58530/2023/1923