1922
Altered brain structure in a mouse model of ALS with mutation in Tardbp is observed from early adulthood1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Mouse Imaging Centre, The Hospital for Sick Children, Toronto, ON, Canada, 3Mammalian Genetics Unit, MRC Harwell Institute, Oxford, United Kingdom, 4Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 5Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 6Neurological Disorders Group, Hospital Clínico San Carlos, IdISSC, Madrid, Spain, 7MRC Prion Unit and Institute of Prion diseases, University College London, London, United Kingdom
Synopsis
Keywords: Neurodegeneration, Preclinical
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by aggregates of TDP-43 in the brain. The TDP-M323K mouse model of ALS has a mutation in Tardbp and presents progressive motor, neurological and behavioural phenotypes, in addition to widespread changes in brain volume at 12 months of age. Here, we assessed if these volumetric changes are progressive or if they are already present before other symptoms start to present. Post-mortem structural MRI in 3- and 12 months-old TDP-M323K mice revealed that brain volume is already altered in young adults despite the absence of major clinical and pathological phenotypes.Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that leads to death usually 3-4 years after diagnosis. Patients present progressive brain imaging alterations such as cortical thinning1 and atrophy of subcortical grey matter2. There is high clinical variability and more than 30 genes are known to cause ALS3. Despite this heterogeneity, more than 98% of ALS patients present aggregates of the TDP-43 protein in the brain4. TDP-43 is an RNA-binding protein with a role in RNA processing and maturation and is encoded by the gene TARDBP (or TDP). Mutations in TARDP account for ~5% of familial ALS5.The TDP-M323K mouse is a model of ALS with an ENU-induced missense mutation in the Tardp gene (p.M323K). Homozygous TDP-M323K mice present altered splicing in target mRNAs and altered expression of multiple genes. Mice develop progressive neurological and motor phenotypes, with grip strength loss at 12 months of age, p62 and ubiquitin inclusions in the spinal cord and brainstem at 18 months of age, and reduction in motor units and motor neuron count in the spinal cord at 24 months of age6. Additionally, early innate behaviour deficit is observed from 3 months of age, and late, progressive anxiety-like phenotype and deficits in associative learning and memory are observed from 12 months of age (data not published).
We have previously described widespread changes in brain structure in 12-month-old homozygous TDP-M323K mice7 that recapitulate alterations observed in human ALS patients8. However, it is not clear if these volumetric changes are progressive, like in ALS patients, or if they are already detectable before other symptoms start to present. In this study, we assessed structural brain phenotypes in post-mortem samples of TDP-M323K mice at 3- and 12 months of age (11- and 54-weeks-old) using high-resolution T2-weighted MRI.
Materials and methods
In total, 29 mice were studied: 6 TDP-M323K homozygous mutants 11-week-old, 7 wild-type littermates 11-week-old, 8 TDP-M323K homozygous mutants 54-week-old, and 8 wild-type littermates 54-week-old (all females, C57BL/6J-DBA/2J background). Mice were perfused with paraformaldehyde (PFA) 4% in 0.1 M PBS under anaesthesia. All perfusion solutions contained 2mM of Gadovist, a Gd-contrast agent (Gd-CA; Bayer Vital GmbH, Leverkusen, Germany). Brains were kept in the skull, fixed overnight in PFA-4%/Gd-CA (2mM), and stored at 4°C in PBS/Gd-CA (2mM)/Na-azide (0.05%) until scanned.Structural MRI was performed at 7.0 tesla. Samples from 54-week-old mice were scanned in a multi-channel 7 Tesla MRI scanner (Agilent Inc., Palo Alto, CA). Sixteen samples were imaged in parallel using a custom-built 16-coil solenoid array9 (T2W 3D FSE cylindrical k-space acquisition sequence10, TR/TE/ETL = 350 ms/12 ms/6, TEeff = 30ms, two averages, FOV/matrix-size = 20 × 20 × 25 mm/504 × 504 × 630, total-imaging-time 14 h). Samples from 11-week-old mice were scanned in a Bruker 7-Tesla 306 mm horizontal bore magnet (BioSpec 70/30 USR, Bruker, Ettlingen, Germany). Eight samples were imaged in parallel using a custom-built 8-coil solenoid array (same scan parameters as for 54-week-old samples, but four effective averages, FOV/matrix-size = 20.2 × 20.2 × 25.2 mm/504 × 504 × 630, total-imaging-time = 13.2 h). For all samples, the resulting anatomical images had isotropic resolution of 40µm voxels.
Images were registered using pydpiper11. Volumes were estimated from the Jacobian determinants and modelled as a function of genotype and age including an interaction term. Differences were considered significant for p<0.05 after false discovery rate (FDR) correction.
Results
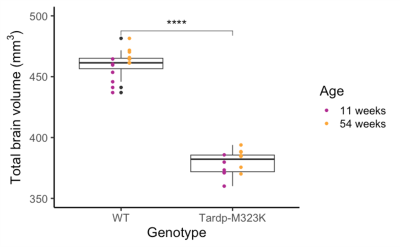
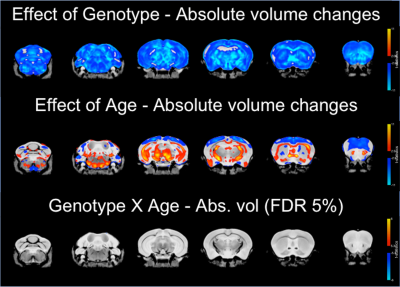
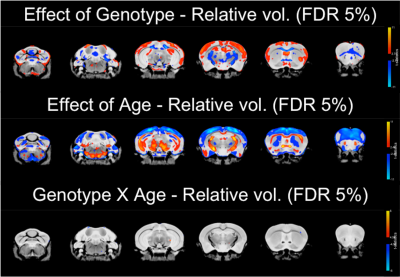
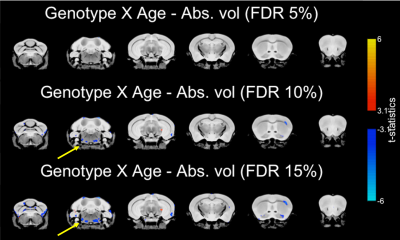
Total brain volume was reduced in TDP-M323K mice (p=4.5e-15) and increased with age (p=0.0012). However, no interaction between genotype and age could be detected in the comparison of total brain volume (p=0.3761, Figure 1).In the voxel-wise analysis, both absolute volume changes and relative volume changes (after removing global scaling differences) were assessed. The effects of genotype, age, and the interaction between genotype and age in absolute volumes are shown in Figure 2, and the same effects in relative volumes are shown in Figure 3. TDP-M323K mice showed a widespread reduction in absolute volume, relative reduction in volume in white matter regions and relative increase in volume in grey matter regions when compared to wild-type littermates. Age led to expected changes in relative volume, in particular reduction of the cortical volume and increase of the white matter volume relative to the whole brain. In this study, we did not observe an interaction between genotype and age in relative volume changes, but a possible interaction between age and genotype was observed at FDR 10%.
Discussion and conclusions
ALS patients present imaging phenotypes that can be detected before clinical symptoms12. However, changes in the volume of brain regions are in general progressive12. Here we have shown that TDP-M323K mice have alterations in brain volume detectable with post-mortem structural MRI since early adulthood. Despite progressive neurological and motor phenotypes, only a few brain regions may present progressive changes in volume observable with this technique, in contrast to what is observed in ALS patients.Acknowledgements
This work was supported by the Wellcome Trust (grant 202788/Z/16/Z), MRC and Harwell funding. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (grant 203139/Z/16/Z).References
1. Verstraete, E. et al. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 83, 383–388 (2012).
2. Westeneng, H.-J. et al. Subcortical structures in amyotrophic lateral sclerosis. Neurobiol. Aging 36, 1075–1082 (2015).
3. van Es, M. A. et al. Amyotrophic lateral sclerosis. Lancet 390, 2084–2098 (2017).
4. Mackenzie, I. R. A., Rademakers, R. & Neumann, M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 9, 995–1007 (2010).
5. Sreedharan, J. et al. TDP-43 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Science (80-. ). 319, 1668–1672 (2008).
6. Fratta, P. et al. Mice with endogenous TDP‐43 mutations exhibit gain of splicing function and characteristics of amyotrophic lateral sclerosis. EMBO J. 37, e98684 (2018).
7. Martins-Bach, A. B. et al. Anatomical and microstructural brain alterations in the TDP-M323K mouse model of amyotrophic lateral sclerosis. in Proc. Intl. Soc. Mag. Reson. Med. 29 1208 (2021).
8. Christidi, F. et al. Gray matter and white matter changes in non-demented amyotrophic lateral sclerosis patients with or without cognitive impairment: A combined voxel-based morphometry and tract-based spatial statistics whole-brain analysis. Brain Imaging Behav. 12, 547–563 (2018).
9. Bock, N. A., Nieman, B. J., Bishop, J. B. & Henkelman, R. M. In vivo multiple-mouse MRI at 7 Tesla. Magn. Reson. Med. 54, 1311–1316 (2005).
10. Spencer Noakes, T. L., Henkelman, R. M. & Nieman, B. J. Partitioning k -space for cylindrical three-dimensional rapid acquisition with relaxation enhancement imaging in the mouse brain. NMR Biomed. 30, e3802 (2017).
11. Nieman, B. J. et al. MRI to Assess Neurological Function. Curr. Protoc. Mouse Biol. 8, e44 (2018).
12. Querin, G., Biferi, M. G. & Pradat, P.-F. Biomarkers for C9orf7-ALS in Symptomatic and Pre-symptomatic Patients: State-of-the-art in the New Era of Clinical Trials. J. Neuromuscul. Dis. 9, 25–37 (2022).
Figures