1919
Blood-brain barrier permeability changes over the lifespan1Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 2Department of Psychology, Center for Lifespan Changes in Brain and Cognition, Oslo, Norway, 3National University of Singapore and National University Health System, Saw Swee Hock School of Public Health, Singapore, Singapore, 4Fraunhofer-Insitute for Digital Medicine MEVIS, Bremen, Germany, 5mediri GmbH, Heidelberg, Germany, 6University College London, Queen Square Institute of Neurology and Centre for Medical Image Computing (CMIC), London, United Kingdom, 7Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany
Synopsis
Keywords: Neurodegeneration, Arterial spin labelling, Blood-brain barrier
Blood-brain-barrier (BBB) dysfunction is a hallmark of aging-related disorders, including cerebral small vessel disease and Alzheimer’s disease. An emerging biomarker of BBB dysfunction is time of exchange (Tex) of water across the BBB as measured by multi-echo arterial spin labeling MRI. We evaluated Tex across the age spectrum in 40 adults from two cohorts of healthy controls, and demonstrated that Tex is higher in gray than in white matter, higher in females than in males, and that Tex decreases with age. These findings suggest that BBB permeability changes over the lifespan can be investigated using arterial spin labeling approaches.Introduction
The blood-brain barrier (BBB) is a cellular structure that protects the brain from toxins. BBB dysfunction not only plays a central role in the aging brain(1,2) but is also potentially one of the earliest microvascular changes in Alzheimer’s disease (AD) and related dementias(3). One of the most common imaging methods for assessing BBB integrity is dynamic contrast-enhanced (DCE) MRI, which relies on the intravenous application of gadolinium-based contrast agents (GBCA). However, DCE has several disadvantages; GBCAs are invasive and costly, and their wide use and potential toxicity are debated. Besides that, GBCAs have higher molecular weights than water and are therefore less sensitive to early subtle BBB changes in water transport through aquaporin-4 (AQP4) water channels, which were associated with aging and with β-amyloid (Aβ) deposits in animal models (4,5) and humans(6). Emerging techniques to image BBB water exchange rate (Tex) are multi-echo (ME) (7) and diffusion-weighted (DW) (8) arterial spin labeling (ASL) – approaches that obviate the need for GBCA injection. ME-ASL has been shown to provide reproducible values of BBB integrity in healthy volunteers (7). Decreased BBB permeability in humans and increased permeability with age in rats were shown previously with DW-ASL (9) and ME-ASL (10), respectively, owing to both differences in the studied population and the principles of the used imaging methods. Here, we investigate Tex changes across age and sex in 40 adults from two cohorts of healthy controls.Methods
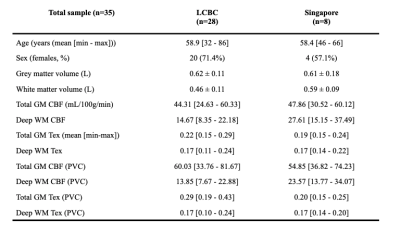
2.1. ParticipantsData from cognitively unimpaired healthy participants were drawn from a population study at the Center for Lifespan Changes in Brain and Cognition (LCBC) at the University of Oslo (28 participants) and from a University of Singapore population study (8 participants).
2.2. Image acquisition and processing
An identical multi-TE, multi-post-labeling-delay (PLD) pCASL protocol utilizing Hadamard-encoding (HAD) was used at two 3T systems (MAGNETOM Prisma, Siemens Healthineers, Erlangen, Germany) with different receiver coils (32-channels-head-coil/Oslo, 64-channels-head-coil/Singapore)(7,11). Two pCASL sequences were acquired: a HAD-8 sequence with a labeling duration (LD) of 400ms, PLD [600:400:3400]ms, and TE 12.5ms. And a HAD-4 sequence with LD 1000ms, PLD 1500, 2500, 3500ms, and 8 TEs [14.4:28.9:217.2]ms. Data were analyzed with ExploreASL(12), and gray (GM) and white matter (WM) CBF and Tex were quantified with and without partial volume correction (PVC)(13) with a modified version of FSL-FABBER(14).
2.3. Statistical analysis
Pearson correlation coefficients with age were assessed for CBF and Tex. The resulting data for GM, CBF, and Tex were statistically evaluated for the groups (female/male) using a t-test. Wilcoxon signed-rank test was used for the non-normally distributed WM values. General linear models were performed to study the associations of CBF and Tex with age and sex, with the site as a covariate.
Results
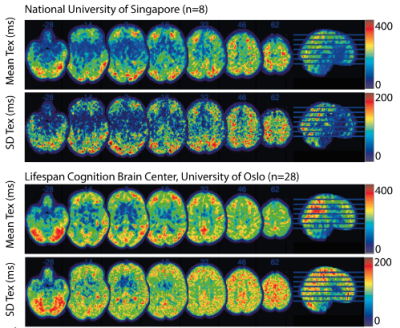
Participants from LCBC (n=28) were between 32-86 years with 71.4% females. These from Singapore (n=8) were between 46-66 years with 57.1% females (Table 1).Both GM and WM CBF were negatively correlated with age, both with and without PVC. GM CBF differed between males and females (p=0.04 – both with and without PVC), which was not the case for WM CBF (p=0.32 with and p=0.17 without PVC). Visually, both site averages (Figure 1) show similar Tex patterns, with relatively high Tex in the cortex and in the posterior flow territory.
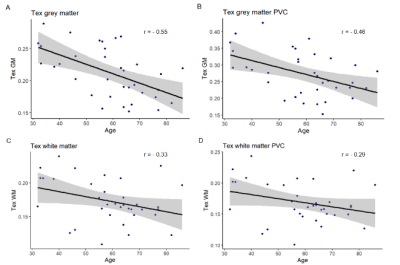
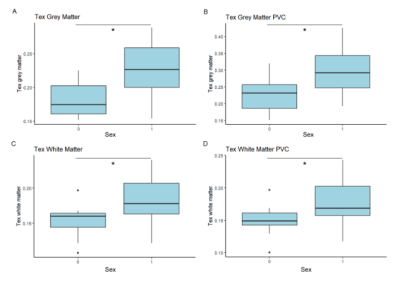
Both GM and WM Tex were negatively correlated with age (Figure 2). GM Tex had a moderate correlation with age, both with and without PVC. As with CBF, the WM Tex correlation with age was lower but statistically significant, both with and without PVC. Additionally, significant differences between males and females were found for GM Tex, both with and without PVC (p=0.001/p=0.003, Figure 3), as well as for WM Tex (p=0.009/p=0.01).
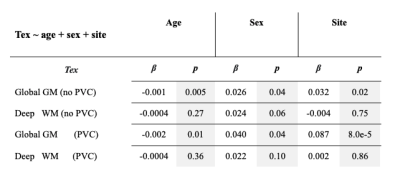
In the more extensive GLM models, GM Tex was negatively associated with age, both with (β=-0.001, p=0.005) and without PVC (β=-0.002, p=0.01), while WM Tex was not significantly associated with age (Table 2). There was a site effect for the GM Tex (β=0.032, p=0.02) that became stronger after PVC (β=0.087, p=0.00008).
Discussion
In addition to site effects, both CBF and Tex were moderately associated with age and significantly differed between sexes, with effects stronger in GM than in WM. Although most attributes were similar between cohorts, differences in the absolute number, male/female ratio, and technical setup might strongly affect these preliminary results. Our aging results are in line with previous studies that showed higher water permeability with aging, but the observed sex differences have not been yet reported. While PVC did not change most of our associations, it had a striking effect on the site effects. This effect could be explained by the contrast differences between ASL and T1w-images available for registration and segmentation. This illustrates the importance of addressing site effects not only in the statistical analysis but also in an image processing pipeline.Acknowledgements
The DEBBIE project (Developing a non-invasive biomarker for early BBB breakdown in Alzheimer’s disease) is an EU Joint Programme -Neurodegenerative Disease Research (JPND) project. For this DEBBIE substudy, we received funding through the following funding organisations under the aegis of JPND -www.jpnd.eu (BMBF in Germany, NFR in Norway, and ZonMw and Alzheimer Nederland in The Netherlands). The project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 825664.References
1. Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019;25:270–276.
2. Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res. 2009;171:232–241.
3. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020;581:71–76.
4. Yang J, Lunde LK, Nuntagij P, et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J. Alzheimers. Dis. 2011;27:711–722.
5. Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76:845–861.
6. Zeppenfeld DM, Simon M, Haswell JD, et al. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017;74:91–99.
7. Mahroo A, Buck MA, Huber J, et al. Robust Multi-TE ASL-Based Blood-Brain Barrier Integrity Measurements. Front. Neurosci. 2021;15:719676.
8. Shao X, Ma SJ, Casey M, D’Orazio L, Ringman JM, Wang DJJ. Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magn. Reson. Med. 2019;81:3065–3079.
9. Ford JN, Zhang Q, Sweeney EM, et al. Quantitative Water Permeability Mapping of Blood-Brain-Barrier Dysfunction in Aging. Front. Aging Neurosci. 2022;14:867452.
10. Ohene Y, Harrison I, Evans PE, Thomas DL, Lythgoe MF, Wells J. Increased blood-brain interface permeability to water in the ageing brain detected using non-invasive multi-TE ASL MRI. Magn. Reson. Med. 2020;In press:In press.
11. Gregori J, Schuff N, Kern R, Günther M. T2-based arterial spin labeling measurements of blood to tissue water transfer in human brain. J. Magn. Reson. Imaging 2013;37:332–342.
12. Mutsaerts HJMM, Petr J, Groot P, et al. ExploreASL: an image processing pipeline for multi-center ASL perfusion MRI studies. Neuroimage 2020:117031.
13. Petr J, Mutsaerts HJMM, De Vita E, et al. Effects of systematic partial volume errors on the estimation of gray matter cerebral blood flow with arterial spin labeling MRI. MAGMA 2018;31:725–734.
14. Chappell MA, Groves AR, Whitcher B, Woolrich MW. Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Trans. Signal Process. 2009;57:223–236.
Figures