1901
Structural and Neuroradiological Findings in COVID-19 Patients with Post-COVID Neurological Symptoms with Ultrahigh Field 7T MRI1Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4General Internal Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Keywords: Infectious disease, COVID-19, Ultrahigh Field, hippocampal subfields, amygdala subnuclei
We present preliminary findings of neuroradiological analysis and FreeSurfer-based cortical segmentation analysis which show changes in deep gray matter structures from ultrahigh field MR neuroimaging at 7T in patients with post-COVID neurological symptoms. 8 hippocampal subfields and 4 amygdala subnuclei had significantly increased volume in a cohort of 12 COVID-19 patients with post-COVID neurological symptoms compared to matched healthy controls. These changes may indicate neuroinflammation of the hippocampus and amygdala in COVID-19, which may be related to the neurological symptoms of memory impairment, “brain fog” and anxiety in our COVID patient cohort.
Introduction
The novel Coronavirus Disease 2019 (COVID-19), which is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is an ongoing highly contagious respiratory infection1 that has infected 638 million and killed 6.6 million people worldwide2. Although most COVID-19 patients recover without any lasting symptoms, a significant proportion of COVID-19 patients continue to experience persistent neurological sequelae3 including stroke, visual defects, anosmia/dysgeusia, brain fog4, among others, which are not well understood. Long-haul COVID-19 syndrome4 is expected to become a serious healthcare burden in the next several years5. A recent large neuroimaging study has demonstrated subtle changes in the brain of COVID-19 patients6. Here, we report our preliminary findings of neuroradiological analysis and changes in deep gray matter structures from ultrahigh field MRI at 7T in patients with post-COVID neurological symptoms.Methods
12 COVID-19 patients (age: 45±14 years , 3 female) who experienced post-COVID neurological symptoms were enrolled, as well as 12 age- and sex-matched healthy control subjects (age: 47±15 years , 3 female). All subjects underwent whole brain MRI on a Siemens Magnetom 7T scanner using a 1Tx/32Rx head coil. The COVID-19-specific MRI protocol included axial T1-weighted MP2RAGE, FLAIR, T2-weighted TSE, susceptibility weighted imaging (SWI), as well as coronal-oblique T2-weighted TSE (imaging plane oriented perpendicular to olfactory bulbs). Images were evaluated by 3 fellowship-trained neuroradiologists for T2/FLAIR hyperintensities (based on the Fazekas scale), infarcts, chronic microhemorrhages and macrohemorrhages, encephalopathy features, brain volume loss (qualitative), olfactory bulb abnormalities and brainstem abnormalities.Cortical image reconstruction and automated segmentation of major brain structures as well as secondary segmentation of the amygdala subnuclei, thalamic subnuclei, hippocampal subfields, and brainstem regions was carried out with FreeSurfer7 (version 7) using T1-weighted MP2RAGE images. Statistical analysis of segmentation volumetrics between COVID-19 patients and healthy controls was carried out in MATLAB using a two-tailed t-test for normally distributed volumes or using a Wilcoxon rank sum test for non-normally distributed volumes. Normality was tested using the Shapiro-Wilk test.
Results
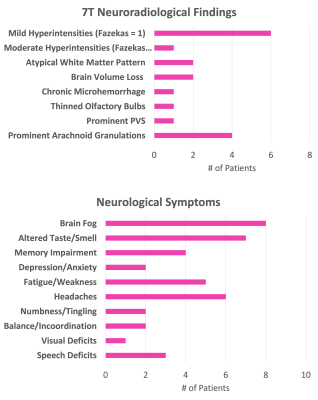
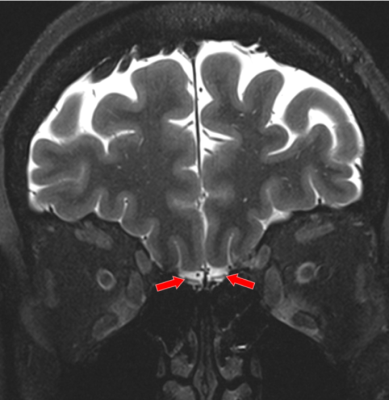
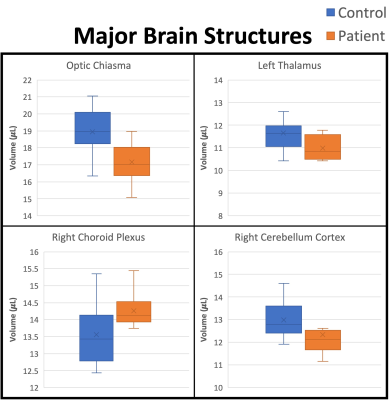
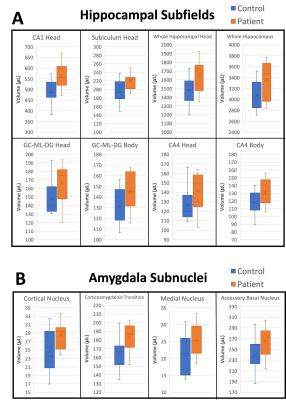
Histograms of the post-COVID neurological symptoms and 7T neuroradiological findings shown in Fig. 1. The neuroradiological findings were heterogeneous across the patients. No infarcts and no encephalopathy features were observed in any patient. Olfactory bulb thinning (Fig. 2) was observed in one patient who presented neurological symptoms of anosmia and dysgeusia. Moderate TSE/FLAIR hyperintensities (Fazekas scale 2) were observed in one patient who presented with several neurological symptoms including COVID long haul syndrome, numbness, headache and blurry vision (Fig. 3). Mild hyperintensities (Fazekas scale 1) were observed in 6 patients. Prominent arachnoid granulations were incidentally found in 4 patients.Volumetric analysis of the major brain regions (Fig. 4) showed that there is a significant decrease in volume in 4 brain regions in the COVID patients compared to controls: optic chiasma (9%), right cerebellum cortex (5%), left thalamus (6%) (p<0.05 for all). COVID patients also had 5% higher volume of the right choroid plexus compared to controls (p<0.05). Secondary segmentation volumetric analysis of the deep brain structures (Fig. 5) showed that there is significant increase in volume in 8 hippocampal subfields in COVID patients compared to controls (p<0.05). The largest increase was in the CA1 head at 14%. Additionally, 4 amygdala subnuclei had increase in volume in COVID patients compared to controls (p<0.05), with the largest increase of 21% in the medial nucleus.
The time between COVID-19 diagnosis and 7T MRI was 16±8 months.
Discussion
In this ongoing study, we report our preliminary neuroradiological findings and brain volumetric segmentation analysis from 7T MRI in 12 patients with post-COVID neurological symptoms. The neuroradiological findings are very heterogeneous. It is interesting that these findings persist even after 16 months post COVID infection. It should be noted that thinning of the olfactory bulbs was observed in one patient, who also reported the neurological symptom of altered taste and smell. It is also interesting that 4 patients presented the incidental finding of prominent arachnoid granulations. This finding, along with the increased volume of the right choroid plexus (which is thought to be an area of CSF production) may indicate lymphatic involvement in the neurotropism of SARS-CoV-2 virus.The volumetric increase of several hippocampal subfields and amygdala subnuclei in the COVID patients is interesting and may indicate neuroinflammation or edematous inflammation in the hippocampus and the amygdala. Neuroinflammatory processes have been reported in COVID-198. The hippocampus is thought to be involved in learning and memory, and inflammation in the hippocampus may be related to memory impairment, which was observed in 4 patients, as well as “brain fog”, which was observed in 8 patients and is widely reported in COVID-19 patients. The amygdala is thought to be involved in processing of emotions such as aggression and fear, and changes in the amygdala may be related to the neurological symptom of anxiety, which was reported in 2 patients in our COVID cohort.
Because of the small sample size of this study, the statistical analyses were not corrected for multiple comparisons. This study is actively ongoing, and we expect to conduct more thorough statistical analyses after accumulating a larger sample size of both COVID-19 patients and age- and sex-matched healthy controls.
Acknowledgements
The authors wish to acknowledge the National Institutes of Health for the following sources of funding support for this study:
NIH NINDS 1R21NS122389-01
NIH NCI 5R01CA202911-05
References
1. Chen N, et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 2020. 395(10223): p. 507-513.
2. Ritchie H, et al., "Coronavirus Pandemic (COVID-19)", 2020, Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/coronavirus on Nov. 2021 [Online Resource]
3. Ladopoulos T, et al., “COVID-19: Neuroimaging Features of a Pandemic”. J Neuroimaging, 2021. 31(2): p. 228-243
4. Asadi-Pooya AA, et al., “Long COVID syndrome-associated brain fog”, J Med Virol. 2021 Oct 21. doi: 10.1002/jmv.27404.
5. Higgins V, et al., “COVID-19: from an acute to chronic disease? Potential long-term health consequences”. Crit Rev Clin Lab Sci. 2021 Aug;58(5):297-310. doi: 10.1080/10408363.2020.1860895. Epub 2020 Dec 21. PMID: 33347790.
6. Douaud G, et al., “SARS-CoV-2 is associated with changes in brain structure in UK Biobank”. Nature. 2022 Apr 28;604(7907):697–707.
7. Fischl B. “FreeSurfer”. NeuroImage. 2012 Aug;62(2):774–781.
8. Langan MT, et al., “Semi-automated Segmentation and Quantification of Perivascular Spaces at 7 Tesla in COVID-19”. Front Neurol. 2022 Apr 1;13:846957.
Figures