1899
Dysfunctional Respiratory Patterns in Symptomatic Post-Acute Covid-19 Patients on Dynamic High Temporal Resolution Free-Breathing Lung MRI1Radiology, NYU Langone Health, New York, NY, United States, 2Siemens Medical Solutions, New York, NY, United States
Synopsis
Keywords: Lung, COVID-19
Dynamic high temporal resolution free-breathing MRI allows for quantification of respiratory duration, depth and rate—parameters separate from those provided by pulmonary function tests. Post-acute Covid patients with persisting cardiopulmonary symptoms demonstrated larger tidal area, longer duration of inspiration, and larger variability in respirations.Introduction
Post-acute sequelae of Covid-19 infection (PASC) has been estimated to develop in up to three-fourths of patients1–3. PASC is comprised of multi-systemic manifestations, with fatigue and dyspnea among the most commonly reported symptoms4. Patients may have an unclear cause or mechanism for these varied symptoms despite diagnostic testing3.Our purpose was to derive and assess respiratory patterns on dynamic high temporal resolution free-breathing chest MRI as a correlate of persisting cardiopulmonary symptoms. We hypothesized patients with reported cardiopulmonary symptoms may be more likely to demonstrate dysfunctional respiratory patterns.
Methods
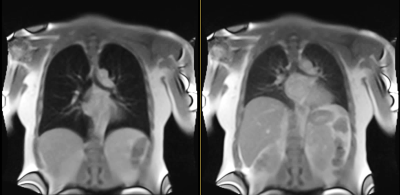
30 MRI exams were acquired in 28 individuals (19 men; mean age 51+15 years) with history of Covid-19 pneumonia, and review of systems assessment within 30 days (6.5+20 days). Imaging was performed on a commercial MRI system (1.5T MAGNETOM Aera; Siemens Healthcare, Erlangen, Germany) modified to operate as a 0.55T prototype system. Exams were acquired in the post-acute Covid-19 phase, at mean of 333+185 days from acute Covid-19 infection. Cardiopulmonary symptoms assessed included chest pain, palpitations, dyspnea, dyspnea on exertion, cough, wheezing, difficulty breathing, and lightheadedness.High temporal resolution dynamic MR data was acquired using a 2D True FISP sequence with a thick coronal slice (15mm) positioned at the level of distal trachea/carina. 14 exams were acquired at temporal resolution of 190ms, FOV 370 x 450 mm2, in-plane resolution 4x4 mm2, flip angle 27°, readout bandwidth 2126 Hz/Px, echo spacing 1ms, NEX 2, and measurement time 51s. 16 exams were acquired at temporal resolution of 208ms, FOV 450x450 mm2, in-plane resolution 1.8x1.8 mm2, flip angle 30°, readout bandwidth 1002 Hz/Px, echo spacing 2.4ms, and measurement time 77s (Figure 1).
An automated 2DxTime lung postprocessing workflow (fireVoxel, www.firevoxel.org) consisted of:
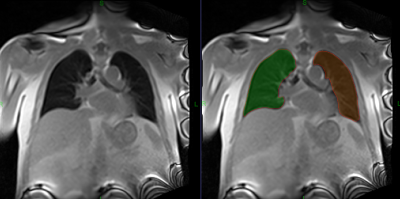
· Segmentation of left and right lung at each of 250 time points (Figure 2). Segmentation accuracy was facilitated by drawing an approximate lung contour on the first frame only.
· Registration of each image to the first time point segmentation using deformable registration method5.
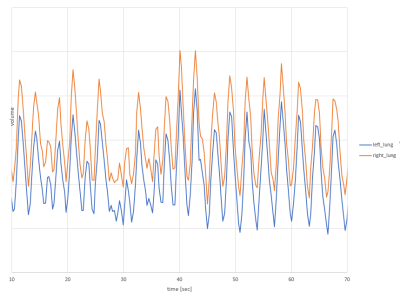
· Construction of respiratory curves for each lung based on segmentation mask area at each time point (Figure 3). Processing of each exam was performed on an AMD-Ryzen-6000 series workstation.
Quantitative parameters were derived for each lung including: minimum and maximum lung area, tidal area, diaphragmatic amplitude, and inspiratory and expiratory rate and duration. Respiratory periodicity (left and right combined) was quantified through incoherence, a novel robust measure of departure of respiratory curves from periodicity.
Quantitative parameters were compared between patients with and without persisting cardiopulmonary symptoms. Two-sided 5% significance level was used.
Results
Mean and standard deviation for tidal area, inspiratory and expiratory duration and rate, and incoherence are provided in Figure 4.Respiratory incoherence was greater in symptomatic than asymptomatic individuals (p=.04). Tidal area was significantly higher in symptomatic than asymptomatic individuals during free breathing (right lung 16.5 versus 11cm2, p=.01; and left lung 14.5 versus 9cm2, p<.001). Duration of each inspiration was longer in symptomatic than asymptomatic individuals (1.7s versus 1.3s in each lung, p=.01).
Inspiratory slope (1/sec) was greater in symptomatic than asymptomatic individuals (right lung .1 versus .07, p=.007; left lung 0.1 versus .08, p-.012). Expiratory slope was greater in symptomatic than asymptomatic individuals (right lung -.08 versus -.05, p=.009; left lung -.08 versus -.05, p=.018).
There was no significant difference between symptomatic and asymptomatic groups in terms of age, gender, body mass index, body surface area, history of hospitalization or intubation, or image protocol.
Discussion
We found post-acute Covid patients with persisting cardiopulmonary symptoms demonstrated larger tidal area, longer duration of inspiration, and greater variability in respirations. Our segmentation tool allowed for derivation of new quantitative respiratory metrics beyond those provided by pulmonary function tests.Dysfunctional breathing refers to altered breathing patterns instigating intermittent or chronic respiratory or non-respiratory symptoms6,7. Incoherence, or departure from purely periodic respiration, in symptomatic patients may reflect quantification of previously described features of dysfunctional breathing, like deep sighs7. Symptomatic patients in our cohort may have demonstrated larger tidal volumes due to feelings of breathlessness or air hunger, which are associated with dysfunctional breathing. Symptomatic patients also demonstrated longer duration of inspiration, in line with taking larger breaths.
To our knowledge, there are limited methods of respiratory pattern quantification. Breathing pattern has been recorded via structured light plethysmography in asthma patients8, and ultrasound may potentially provide limited quantitative metrics in relation to diaphragmatic motion.
Dynamic MRI provides several advantages as a tool for quantifying respiratory patterns. First, this is a non-invasive and fast exam, with the sequence acquired in under 80 seconds. Second, patient instruction is not required, in contradistinction to pulmonary function tests. We showed even metrics acquired during tidal breathing proved salient. And finally, a variety of information can be assessed including respiratory rate, depth, duration, and periodicity—in addition to lung area. Future developments can investigate multi-slice acquisitions, instructed deep breathing maneuvers, upright MRI9, regional lung analysis, as well as lung deformation/elasticity10.
Conclusion
Dynamic high temporal resolution free-breathing MRI allows for quantification of respiratory duration, depth, rate and pattern—parameters separate from those provided by pulmonary function tests. Post-acute Covid patients with persisting cardiopulmonary symptoms demonstrated larger variability in respirations, larger tidal area, and longer duration of inspiration.Acknowledgements
Grant support: NIH P41EB017183References
1. Yoo SM, Liu TC, Motwani Y, Sim MS, Viswanathan N, Samras N, Hsu F, Wenger NS. Factors Associated with Post-Acute Sequelae of SARS-CoV-2 (PASC) After Diagnosis of Symptomatic COVID-19 in the Inpatient and Outpatient Setting in a Diverse Cohort. J Gen Intern Med 2022;37:1988–1995.
2. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet 2021;397:220–232.
3. Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, Raza H, Okeke O, Dewar RL, Higgins BP, Tolstenko K, Kwan RW, Gittens KR, Seamon CA, McCormack G, Shaw JS, Okpali GM, Law M, Trihemasava K, Kennedy BD, Shi V, Justement JS, Buckner CM, Blazkova J, Moir S, Chun T-W, Lane HC. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann Intern Med 2022;175:969–979.
4. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis 2022;226:1593–1607.
5. Heinrich MP, Jenkinson M, Sir Michael Brady, Schnabel JA. Globally optimal deformable registration on a minimum spanning tree using dense displacement sampling. Med Image Comput Comput-Assist Interv MICCAI Int Conf Med Image Comput Comput-Assist Interv 2012;15:115–122.
6. Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 2016;25:287–294.
7. Barker N, Everard ML. Getting to grips with ‘dysfunctional breathing’. Paediatr Respir Rev 2015;16:53–61.
8. Sakkatos P, Bruton A, Barney A. Changes in quantifiable breathing pattern components predict asthma control: an observational cross-sectional study. Asthma Res Pract 2021;7:5.
9. Daentzer D, Venjakob E, Schulz J, Schulze T, Schwarze M. Influence of microsurgical decompression on segmental stability of the lumbar spine - One-year results in a prospective, consecutive case series using upright, kinetic-positional MRI. BMC Musculoskelet Disord 2022;23:742.
10. Chassagnon G, Martin C, Marini R, Vakalopolou M, Régent A, Mouthon L, Paragios N, Revel M-P. Use of Elastic Registration in Pulmonary MRI for the Assessment of Pulmonary Fibrosis in Patients with Systemic Sclerosis. Radiology 2019;291:487–492.
Figures