1888
Machine learning methods for cerebral perfusion status prediction1School of Biological Science and Medical Engineering,Beihang University, Beijing, China, 2National Space Science Center,Chinese Academy of Sciences, Beijing, China, 3Department of Ultrasound, Beijing Friendship Hospital, Beijing, China, 4Department of Radiology, Beijing Friendship Hospital, Beijing, China
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Machine Learning/Artificial Intelligence
The diagnosis and evaluation of cerebral perfusion status are crucial for the management of brain diseases. However, the detection method of cerebral perfusion status is complicated. Considering that CBF is mainly supplied by the internal carotid artery (ICA), this paper proposes a novel cerebral perfusion status prediction model, which can automatically quantify the cerebral perfusion level of patients by modeling the association between ICA blood flow and cerebral perfusion. The experimental results on a real-world dataset using machine learning methods can achieve satisfactory performance. Thus, it can be used as an effective adjuvant tool for determining the cerebral perfusion status.Introduction
Abundant and uninterrupted cerebral blood flow (CBF) or perfusion is fundamental for maintaining metabolic and positive physiological functions1.Most cerebral perfusion imaging techniques use large equipment such as computed tomography (CT) and magnetic resonance imaging (MRI) systems. However, these examination devices are complicated to operate, need to be controlled by specialized technicians. These issues make it challenging to adopt cerebral perfusion imaging techniques in some special scenarios.The brain is main supplied with blood the internal carotid artery (ICA) system, and distribution of total CBF is approximately 72% in the anterior circulation2. Therefore, ICA blood flow can reflect CBF to some extent and provides a basis for predicting cerebral perfusion status.Machine learning (ML) has recently generated considerable interest in the predictive modeling of human physiological states. In this paper, we used dobutamine to simulate ICA blood flow changes and quantizing the CBF using 3D pseudocontinuous arterial spin labeling (3D-pcASL)3 to create a dataset. The correlation between ICA blood flow and cerebral perfusion status was established using ML methods.Methods
A total of 48 participants (43 males and 5 females) were finally enrolled in the study after screening. To explore the effect of changes in ICA blood flow on cerebral perfusion, this study used intravenous administration of dobutamine to mimic changes in ICA blood flow in volunteers. The experimental process is shown in the Fig. 1.To construct the cerebral perfusion prediction model, the difference between the physiological and neck hemodynamic parameter characteristics in the resting and load states and the demographic characteristics are used as inputs to the machine learning model, and the range of cerebral perfusion state changes, i.e., the percentage performance of cerebral blood flow increment: (CBF in resting state - CBF in load state) / CBF in resting state, is used as the prediction results. 15% was used as a classification threshold in this paper under the guidance of clinicians. The method includes data preparation and preprocessing, feature extraction and model construction.[data preparation and preprocessing] The ICA blood flow data were collected using portable pulse-wave Doppler ultrasonography (SonoscapeX5). The measurement position was 1.5 cm at dilatation, the probe frequency was set to 5−10 MHz. The ICA data were preprocessed by eliminating the improperly collected data and the data corrupted by strong noise.The brain MRI data were acquired using 3D-pcASL, an MR technique that uses water molecules in the blood as an endogenous, freely diffusible tracer for imaging the blood flow in the brain. This study used a 3.0T MRI scanner (Ingenia 3.0T; Philips Healthcare, Best, Netherlands) with a commercial body coil for transmission and a 16-channel head coil for the reception. Images were acquired with the following parameters: repetition time [TR] = 3903 ms, echo time [TE] = 12 ms, post labelling delay (PLD) = 1500 ms, label distance = 90mm, slice thickness = 3.5 mm, number of slices = 20, field of view (FOV) = 240 mm×180 mm, matrix = 64×47, and number of excitations (NEX) = 2.Vital signs monitor (Mindray, BeneVision N12) was used to collect physiological data, including heart rate (HR) and blood pressure (BP).[feature extraction] In this study, 10 features were extracted based on ICA data and physiological data. First, the ultrasound hemodynamic features including peak systolic flow velocity (Vs), end-diastolic flow velocity (Vd), mean blood flow velocity (Vm), resistance index (RI), pulsatility index (PI), systolic/diastolic ratio (S/D) and accelerated speed (AS) were extracted based on ICA data. The extracted features based on vital sign data included: HR, SBP and DBP, and demographic features including subject's age (26.3±3.2, years) and BMI (22.7±2.6,).[ML model construction]In this paper, cerebral perfusion state prediction models are developed using a variety of machine learning methods including support vector machine (SVM)4, k-nearest neighbor (KNN)5, decision tree (DT)6, and three ensemble learning models including random forest (RF)7, gradient boosting decision tree (GBDT)8, and extreme gradient boosting (XGBoost)9. To avoid the randomness brought by cross-validation, we repeated 5 cross-validations 10 times and took the average value as the final result.
Results and Discussion
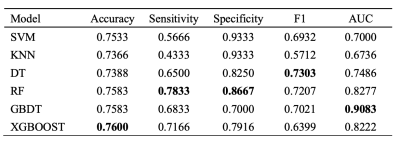
To determine the most appropriate model for predicting cerebral perfusion status, extensive experiments were conducted to validate the performance of the machine learning approach, and the experimental results are shown in the Fig.2, where the best results for each evaluation metric are shown in bold. As can be seen, the accuracy of all algorithms can be maintained above 73.88%, indicating that the classification results match the actual state better.In this study, we systematically investigated the performance of six machine learning models in cerebral perfusion state prediction and used five-fold cross-validation to obtain a more performant and stable prediction model.
Conclusion
Our study is the first to develop machine learning models that predicts cerebral perfusion status based on ICA blood flow. The model can provide clinical aids for physicians to make decisions, working to provide higher quality care to high-cost populations at a lower cost.Acknowledgements
This work was supported by the Beijing Scholar 2015 (Zhenchang Wang), Beijing key Clinical Discipline Funding (No. 2021-135), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (No. ZYLX202101), and Space Medical Experiment Project of China Manned Space Program (No. HYZHXM01012).References
1. Lie, Sole Lindvåg, Jonny Hisdal, and Lars Øivind Høiseth. 2021. “Cerebral Blood Flow Velocity during Simultaneous Changes in Mean Arterial Pressure and Cardiac Output in Healthy Volunteers.” European Journal of Applied Physiology 121(8):2207–17. doi: 10.1007/s00421-021-04693-6.
2. Gofur, Ekramul M., and Bruno Bordoni. 2022. Anatomy, Head and Neck, Cerebral Blood Flow.
3. Järnum, H., Steffensen, E.G., Knutsson, L., Fründ, E.-T., Simonsen, C.W., Lundbye-Christensen, S., Shankaranarayanan, A., Alsop, D.C., Jensen, F.T., Larsson, E.-M., (2010). Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 52, 307–317. https://doi.org/10.1007/s00234-009-0616-6
4. Cortes C, Vapnik V. Support-vector networks. Mach Learn 1995;20:273–97. https://doi.org/10.1007/BF00994018.
5. Cover T, Hart P. Nearest neighbor pattern classification. IEEE Trans Inf Theory 1967;13:21–7. https://doi.org/10.1109/TIT.1967.1053964.
6. Prasad AM, Iverson LR, Liaw A. Newer Classification and Regression Tree Techniques: Bagging and Random Forests for Ecological Prediction. Ecosystems 2006;9:181–99. https://doi.org/10.1007/s10021-005-0054-1.
7. Breiman L. Random Forests. Mach Learn 2001;45:5–32. https://doi.org/10.1023/A:1010933404324.
8. Liu W, Fan H, Xia M. Credit scoring based on tree-enhanced gradient boosting decision trees. Expert Syst Appl 2022;189:116034. https://doi.org/10.1016/j.eswa.2021.116034.
9. Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. Proc. 22nd ACM SIGKDD Int. Conf. Knowl. Discov. Data Min., New York, NY, USA: ACM; 2016, p. 785–94. https://doi.org/10.1145/2939672.2939785.