1886
Prediction of ductal carcinoma in situ with microinvasion postoperatively in women with biopsy-confirmed ductal carcinoma in situ1Department of Radiology, The First Affiliated Hospital of Soochow University, Suzhou, China, 2Department of Radiology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, China
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Breast
To predict ductal carcinoma in situ with microinvasion (DCISMI) based on clinicopathologic, conventional breast magnetic resonance imaging (MRI), and dynamic contrast enhanced MRI (DCE-MRI) radiomics signatures in women with biopsy-confirmed ductal carcinoma in situ (DCIS) to choose high-risk women who may benefit from sentinel lymph node biopsy at initial surgery. The mixed model showed better AUC values than both clinicopathological and DCE-MRI radiomics models in both training/test sets with heterogeneous enhancement and radiomics scores as significant independent predict factors. The mixed model showed the greatest overall net benefit for upstaging and the second was the combine model.
Keywords
Ductal carcinoma in situ, Ductal carcinoma in situ with microinvasive, MRI, Clinicopathologic, Radiomics, UpstageIntroduction
With medical advances, the number of patients with ductal cancer in situ (DCIS) and DCIS with microinvasion (DCISMI) is increasing1. Malignant epithelial cell growth inside the mammary duct lumen but no penetration beyond the basement membrane is the histological hallmark of DCIS2. DCISMI is thought to be the transitional stage between DCIS and invasive ductal cancer (IDC)3. Clinically, the majority of DCIS and DCISMI exhibit a clinically comparable morphological appearance. However, a large-scale clinical study4 revealed that the prognosis of DCISMI is more like that of small invasive carcinoma than DCIS. A radiomics-based signature could provide a more thorough approach by combining both geographical and temporal data to define the tumor more fully. Dynamic contrast enhanced MRI (DCE-MRI) offers the best sensitivity for identifying DCIS or DCIS coupled with invasive cancer5.We speculate that radiomics characteristics obtained from DCE-MRI may represent cellular and molecular data and may be able to foretell upstaging in females with biopsy-proven DCIS. In this study, we aimed to compare the performance of clinical-pathological characteristics, conventional breast MRI features, DCE-MRI radiomics signatures, and combined multiple feature models in predicting DCISMI.Methods
The research cohort consisted of 86 individuals with 87 breast lesions (Figure 1). Two 3.0 T scanning systems, each with an eight-channel breast-specific coil, were used for all breast MRI scans. The detailed scanning parameters were summarized in Table 1. Prior to surgery, an ultrasound-guided needle biopsy was completed on each patient. The clinical-pathological including age, body mass intensity (BMI), DCIS grades of the biopsy specimen, the status of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), Ki-67 proliferation, p63 and axillary lymph node metastasis. Conventional MRI features were included lesion side, lesion number, lesion size on DCE-MRI scan (≥ 2cm, < 2cm), and morphologic feature at T2WI-FS and DCE-MRI according to the Breast Imaging Reporting and Data System MRI lexicon. Medical picture registration was done using ITK-SNAP programmed software (version 3.4.0; http://www.itksnap.org). PyRadiomics (version 3.0.1; http://github.com/Radiomics/pyradiomics) was used to extract radiomics features of DCE-MRI. The workflow is presented in Figure 2. The clinicopathologic, conventional MRI, DCE-MRI radiomics, combine (including conventional MRI and DCE-MRI radiomics), traditional (including clinicopathologic and conventional MRI) and mixed (including clinicopathologic, conventional MRI and DCE-MRI radiomics) models were constructed by the logistic regression (LR) classifier with a 3-fold cross-validation. To evaluate the predictive ability of different models, the area under the curve (AUC) of the receiver operating curve (ROC), sensitivity (SEN), specificity (SPE) with 95% confidence interval (CI), accuracy (ACC), positive predictive value (PPV), and negative predictive value (NPV) were calculated. Calibration curves were employed to assess the predictive performance of each model. To evaluate each model's clinical applicability, decision curve analysis (DCA) was used.Results
Among the 87 biopsy-confirmed DCIS lesions, 42 (48.28%) were found to be pure DCIS in the final surgical pathology, and 45 (51.72%) were upgraded to DCISMI. Higher nuclear grade (P = 0.0006), negative p63 (P = 0.0004), negative ER (P = 0.0191), and negative PR (P = 0.0074) were more common in the DCISMI group. The DCISMI group tended to show more peritumoral edema (P = 0.0022) and heterogeneous enhancement (P < 0.0001) frequent than the DCIS group. The prediction performance of the six models was shown in Table 2 and Fig. 3a~3b. The mixed model showed higher AUC than both the clinicopathologic and DCE-MRI radiomics models in both the training (P = 0.0003, 0.0226) and test (P = 0.0332, 0.0391) sets as well. The calibration curves of the models are shown in Fig. 3c, which shows good calibration. DCA (Fig. 3d) illustrated that the mixed model showed the greatest overall net benefit for upstage and the second was the combine model within reasonable threshold probabilities. The significant independent factors in the mixed model were heterogeneous enhancement (OR 33.327, 95%CI 2.317-479.287) and radiomics score (OR 1106.221, 95%CI 10.104-121118.327).Discussion
Our preliminary analysis showed that the preoperative clinicopathologic, conventional breast MRI and DCE-MRI radiomics features could predict the preoperative histological upstage of DCIS. The mixed model showed excellent predictive performance, which may represent an excellent tool to distinguish DCISMI from DICS preoperatively. A change in diagnosis from pure DCIS before surgery to DCISMI after surgery creates a great deal of patient anxiety and possibly a second surgery6. Therefore, an accurate prediction of the histological DCISMI could help with preoperative risk stratification and the best choice of patients who could benefit from more extensive surgery while avoiding overtreatment of patients at low risk. The mixed model established by the LR classifier showed that heterogeneous enhancement pattern and radiomics score were independent predictors of upstage. DCISMI lesions showed more heterogeneous enhancement, which is partly consistent with previous studies7-9. Radiomics is a precision medical method for non-invasive diagnosis, evaluation of efficacy, and biological behavior10. As there was no other DCE-MRI radiomics studies predicting DCISMI preoperatively, our preliminary results should be validated by additional external independent data sets.Conclusion
Our preoperative mixed model with breast clinicopathologic, conventional MRI, and DCE-MRI radiomics signatures enabled a more accurate prediction of upstaging in women with biopsy-proven DCIS.Acknowledgements
None.References
1. Badve SS, Gökmen-Polar Y. Ductal carcinoma in situ of breast: update 2019. Pathology 2019;51(6):563-69. doi: 10.1016/j.pathol.2019.07.005 [published Online First: 2019/09/02]
2. Burstein HJ, Polyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med 2004;350(14):1430-41. doi: 10.1056/NEJMra031301 [published Online First: 2004/04/09]
3. Okumura Y, Yamamoto Y, Zhang Z, et al. Identification of biomarkers in ductal carcinoma in situ of the breast with microinvasion. BMC Cancer 2008;8:287. doi: 10.1186/1471-2407-8-287 [published Online First: 2008/10/08]
4. Sopik V, Sun P, Narod SA. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res Treat2018;167(3):787-95. doi: 10.1007/s10549-017-4572-2 [published Online First: 2017/11/10]
5. Tajima CC, de Sousa LLC, Venys GL, et al. Magnetic resonance imaging of the breast: role in the evaluation of ductal carcinoma in situ. Radiol Bras2019;52(1):43-47. doi: 10.1590/0100-3984.2018.0058 [published Online First: 2019/02/26]
6. Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg 2005;200(4):516-26. doi: 10.1016/j.jamcollsurg.2004.11.012 [published Online First: 2005/04/05]
7. Lee CW, Wu HK, Lai HW, et al. Preoperative clinicopathologic factors and breast magnetic resonance imaging features can predict ductal carcinoma in situ with invasive components. Eur J Radiol 2016;85(4):780-89. doi: 10.1016/j.ejrad.2015.12.027 [published Online First: 2016/03/15]
8. Yoon GY, Choi WJ, Cha JH, et al. The role of MRI and clinicopathologic features in predicting the invasive component of biopsy-confirmed ductal carcinoma in situ. BMC Med Imaging 2020;20(1):95. doi: 10.1186/s12880-020-00494-z [published Online First: 2020/08/14]
9. Wu P, Cui L, Guo BH, et al. [Values of Minimal Apparent Diffusion Coefficient,Difference between Ratios of Apparent Diffusion Coefficients,and Dynamic Contrast-enhanced Magnetic Resonance Imaging Features in Diagnosing Breast Ductal Carcinoma In Situ with Microinvasion]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2019;41(6):737-45. doi: 10.3881/j.issn.1000-503X.11066 [published Online First: 2020/01/08]
10. Alderson PO, Summers RM. The Evolving Status of Radiomics. J Natl Cancer Inst 2020;112(9):869-70. doi: 10.1093/jnci/djaa018 [published Online First: 2020/02/06]
Figures
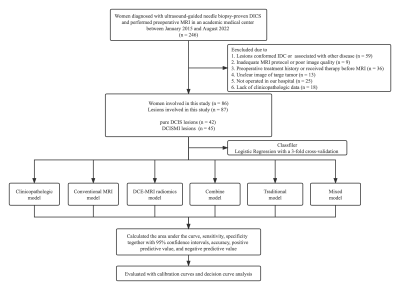
Fig. 1 The flow diagram of the study.
Abbreviations: DICS, ductal carcinoma in situ; DCISMI, ductal carcinoma in situ with microinvasive; IDC, invasive ductal carcinoma; MRI, magnetic resonance imaging; DCE-MRI: dynamic contrast enhanced MRI.
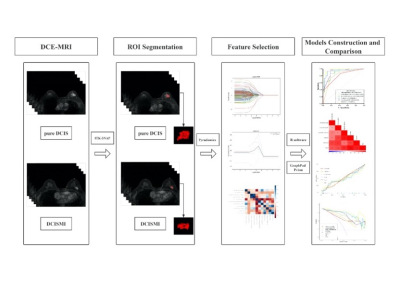
Fig. 2 The radiomics flow chart of the study.
Abbreviations: DICS, ductal carcinoma in situ; DCISMI, ductal carcinoma in situ with microinvasive; MRI, magnetic resonance imaging; DCE-MRI: dynamic contrast enhanced MRI.
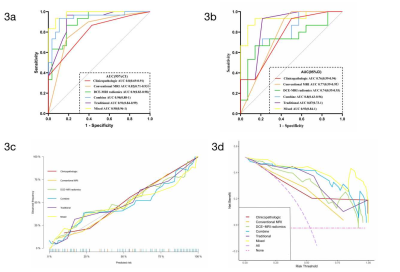
Fig. 3 The receiver operating characteristic curves (ROC), calibration curves and decision curve analysis (DCA) of the six models in the upstaging of DICS.
a The ROC curves of the six models based on Logistic Regression algorithm in the training cohort. b The ROC curves of the six models based on Logistic Regression algorithm in the test cohort. c Calibration curves for the six models based on the Logistic Regression algorithm. d The DCA for the six models based on the Logistic Regression algorithm.
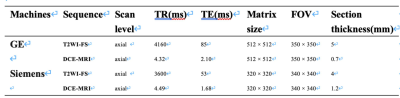
Table 1 MRI sequence scan parameters of the two machines.
Abbreviations: TR, repetition time; TE, echo time; FOV, field of view.
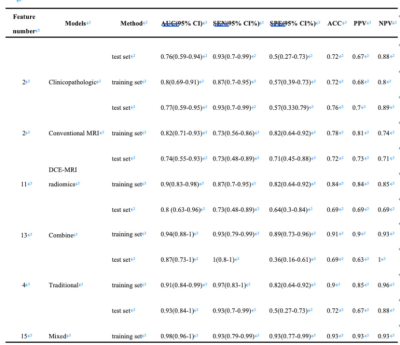
Table 2 Predictive performances of the six models in predicting DCISMI.
Abbreviations: DCISMI, ductal carcinoma in situ with microinvasive; AUC, area under the curve; SEN, sensitivity; SPE, specificity; ACC, accuracy; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; DCE-MRI, dynamic-contrast enhanced MRI.