1876
Magnetic Resonance Imaging-based Radiomics for Clinically Significant Prostate Cancer in PSA Gray Zone to Reduce Unnecessary Biopsies1Department of radiology, First Affiliated Hospital of Soochow University, Suzhou, China, 2MR Scientific Marketing, Siemens Healthineers Ltd, Shanghai, China
Synopsis
Keywords: Prostate, Radiomics, Clinically significant prostate cancer; Prostate-specific antigen
Radiomics was a promising method for csPCa with PSA levels in the gray zone and outperformed PI-RADS assessments.Introduction
Magnetic resonance imaging (MRI) is currently the optimum imaging technology for the diagnosis of clinically significant prostate cancer [1]. The performance of biparametric MRI (bp-MRI), including only T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI) was comparable to that of multiparametric MRI (mp-MRI), which has been proven in previous studies [2,3]. In addition to MRI, prostate specific antigen (PSA) is commonly used in prostate cancer screening with high false positives, especially in men with a PSA level of 4-10 ng/mL [4-6]. Radiomics is an emerging method with potential applications in csPCa diagnosis [7,8]. The clinical value of radiomics based on bp-MRI in predicting csPCa with a PSA level of 4-10 ng/mL is not clear. Therefore, this study aimed to develop and validate a radiomics model based on biparametric magnetic resonance imaging (bpMRI) to predict clinically significant prostate cancer (csPCa) in patients with prostate specific antigen (PSA) levels of 4–10 ng/mL and compare the bpMRI-based radiomics model with prostate imaging reporting and data system (PI-RADS) assessment by radiologists with various experience.Methods
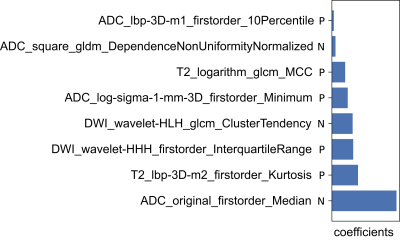
A total of 286 patients were included in this retrospective study and grouped randomly into a training group (n =200) and a test group (n = 86) in a ratio of 7:3. Optimal radiomics features were selected by using analysis of variance (ANOVA), Kruskal-Wallis (KW), Recursive feature elimination (RFE) and least absolute shrinkage and selection operator (LASSO) regression with 10-fold cross-validation in the training group. Logistic regression was used to develop the radiomics model. PI-RADS assessments were performed by an experienced radiologist with 15 years of prostate MRI experience (PI-RADS-e) and radiologists with 2-5 years of MRI experience during clinical routine (PI-RADS-r). The receiver operating characteristic (ROC) curve and decision curve analysis (DCA) were used to evaluate the diagnostic performance of the radiomics model and PI-RADS assessments. The calibration curve was used to evaluate the calibration ability of the radiomics model.Results
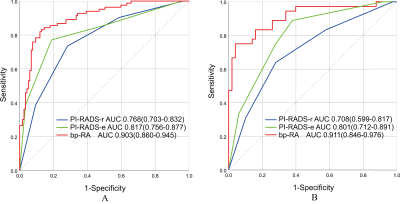
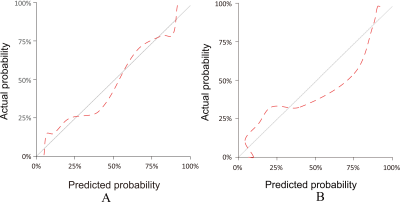
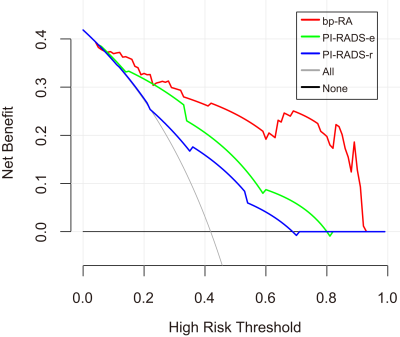
The AUCs of the bpMRI radiomics model (bp-RA), PI-RADS-e and PI-RADS-r were 0.903, 0.817 and 0.768 in the training group, and 0.911, 0.801 and 0.708 in the test group, respectively. The bp-RA had significantly better diagnostic performance than PI-RADS-e and PI-RADS-r (in training group: P=0.011, P<0.001; in test group: P=0.02, P<0.001). The calibration curves showed that bp-RA had good diagnostic ability and the DCA showed bp-RA had higher potential net benefits than PI-RADS assessments by radiologists with various experiences.Conclusions
The radiomics models based on bpMRI were valuable to the detection of csPCa in patients with PSA levels in the gray zone and outperformed PI-RADS assessments by radiologists with various experiences, which can effectively reduce unnecessary biopsies.Acknowledgements
The authors thank all those who helped us during the writing of this research. We also thank the Department of Urology and Pathology of the hospitals for their valuable help and feedback. And we would like to thank the anonymous reviewers for their helpful remarks.References
[1] Monni F, Fontanella P, Grasso A, et al. Magnetic resonance imaging in prostate cancer detection and management: a systematic review[J]. Minerva Urol Nefrol, 2017, 69(6): 567-578. DOI:10.23736/s0393-2249.17.02819-3.
[2] Tamada T, Kido A, Yamamoto A, et al. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2.1[J]. Journal of Magnetic Resonance Imaging, 2021, 53(1): 283-291. DOI:10.1002/jmri.27283.
[3] Di Campli E, Pizzi A D, Seccia B, et al. Diagnostic accuracy of biparametric vs multiparametric MRI in clinically significant prostate cancer: Comparison between readers with different experience[J]. European Journal of Radiology, 2018, 101: 17-23. DOI:10.1016/j.ejrad.2018.01.028.
[4] Van Poppel H, Roobol M J, Chapple C R, et al. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021[J]. Eur Urol, 2021, 80(6): 703-711. DOI:10.1016/j.eururo.2021.07.024.
[5] Aminsharifi A, Howard L, Wu Y, et al. Prostate Specific Antigen Density as a Predictor of Clinically Significant Prostate Cancer When the Prostate Specific Antigen is in the Diagnostic Gray Zone: Defining the Optimum Cutoff Point Stratified by Race and Body Mass Index[J]. J Urol, 2018, 200(4): 758-764. DOI:10.1016/j.juro.2018.05.016.
[6] Gershman B, Van Houten H K, Herrin J, et al. Impact of Prostate-specific Antigen (PSA) Screening Trials and Revised PSA Screening Guidelines on Rates of Prostate Biopsy and Postbiopsy Complications[J]. Eur Urol, 2017, 71(1): 55-65. DOI:10.1016/j.eururo.2016.03.015.
[7] Rodrigues A, Santinha J, Galvao B, et al. Prediction of Prostate Cancer Disease Aggressiveness Using Bi-Parametric Mri Radiomics[J]. Cancers, 2021, 13(23): 17. DOI:10.3390/cancers13236065.
[8] Perez I M, Merisaari H, Jambor I, et al. Detection of Prostate Cancer Using Biparametric Prostate MRI, Radiomics, and Kallikreins: A Retrospective Multicenter Study of Men With a Clinical Suspicion of Prostate Cancer[J]. Journal of Magnetic Resonance Imaging, 2022, 55(2): 465-477. DOI:10.1002/jmri.27811.
Figures