1866
Estimated quantitative relaxation mapping calculated from Multiple-Repetition Multiple-Echo (MRME) based DWI acquisition in prostate1Philips Japan, Ltd., Tokyo, Japan, 2Department of Radiology, Kawasaki Medical School, Kurashiki, Japan, 3Department of Diagnostic Radiology, Radiolonet Tokai, Nagoya, Japan, 4Philips Healthcare, Business Unit MR, Tokyo, Japan
Synopsis
Keywords: Prostate, Quantitative Imaging
It has been reported that synthetised DW images with short TR and zero TE, generated from quantitative relaxation parameters, improved lesion contrast in prostate. In this study, we investigate the added value of the estimated T1 and T2 values in differentiating benign from malignant lesion in suspicious cancers with PI-RADS category 3, potentially reducing unnecessary biopsies. In addition to the conventional DW images at b-values of 1000 and 2000, ADC map, and estimated T1 and T2 maps, synthetised DW images can be acquired in approximately 4 minutes, an acceptable scan time in clinical practice.Introduction
Multiparametric magnetic resonance imaging (mp-MRI) based on Prostate Imaging Reporting and Data System (PI-RADS)1 is increasingly being used in prostate. However, one of the challenges in current prostate MRI is that mp-MRI still does not have sufficient diagnostic accuracy2-5. One article reported that more than half of the lesions with PI-RADS category 3 are not cancerous6. Another article reported approximately 70% of the lesions with PI-RADS category 3 are not cancerous7. These high false-positive rates can lead to unnecessary biopsies. Therefore, improvement of diagnostic accuracy is needed.Synthetic MRI, which synthesizes images using quantitative relaxometry parameters such as T1, T2, and PD, has gained attention and shown promising results, mainly in neuro diseases8, 9. Recently, we have reported clinical usefulness of an arbitrary diffusion contrast-weighted image of the target TR as well as target TE in prostate, based on Multiple-Repetition Multiple-Echo (MRME) based DWI acquisition10. Synthetization of DW images generated with TR of 1000 ms and TE of 0 ms improved lesion contrast in prostate10, utilizing the specific feature that T1 and T2 are shorter in prostate cancer (PCa) than normal tissue11-13. Synthetic images are basically generated from quantitative relaxation parameters. Therefore, these relaxation parameters calculated from MRME-DWI could also be utilized to detect PCa, but to our knowledge, such a study in prostate has not yet been reported. While we know that the quantitative values calculated are only estimates, due to the desire to keep the scan time limited, in this study we validated if multiparametric quantitative maps based on these estimated T1 and T2 can bring added value to differentiate cancer from noncancer in suspicious lesions with PI-RADS category 3.
Methods
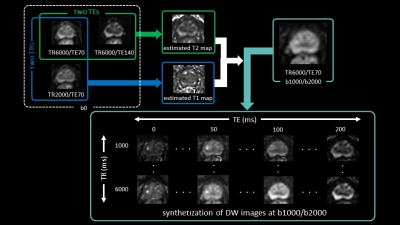
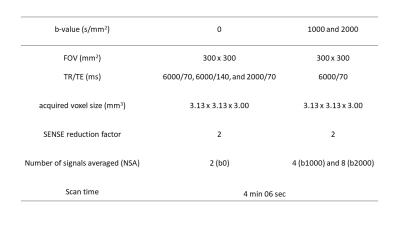
Our Institutional Review Board approved this retrospective study and waived the need for informed consent. Twenty-eight patients with PI-RADS category 3 were evaluated among those who underwent prostate mp-MRI and subsequent MRI-ultrasound fusion-guided prostate-targeted biopsy for lesions suggestive of PCa on mp-MRI between October 2021 and August 2022. All MR images were obtained with a 3T MRI (Ingenia Elition 3.0T; Philips Healthcare, Best, The Netherlands) using an anterior coil and built-in posterior coil. For synthetization of DW image, MRME-DWI acquisition includes b0 images acquired with two different TR and TE, and b1000 and b2000 acquired with single TR and TE (Figure 1). The detailed imaging parameters are described in Figure 2.The T1 can then be estimated by using the signal from 2 b0 images, varying TR and keeping TE constant, while T2 can be estimated from 2 b0 images with constant TR but varying TE.
Estimated T1, T2, and ADC calculated from images at b-value of 0 and 1000 s/mm2 (ADC0-1000) values were compared between PI-RADS category 3 target biopsy-proven cancers and noncancers by using Mann–Whitney U test. Logistic regression was used to assess estimated T1, T2, and ADC0-1000 in the differentiation of cancers versus noncancers. Receiver operating characteristic (ROC) curves and areas under the ROC curve (AUC) were obtained from ordinary logistic regression of the binary outcome. P < 0.05 was considered to indicate a statistically significant difference.
Results and Discussion
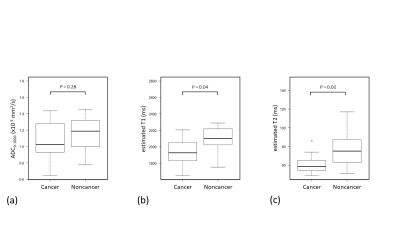
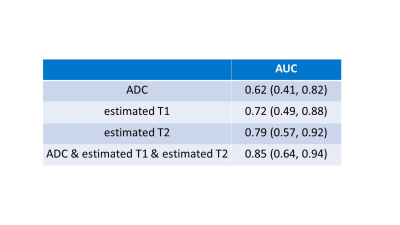
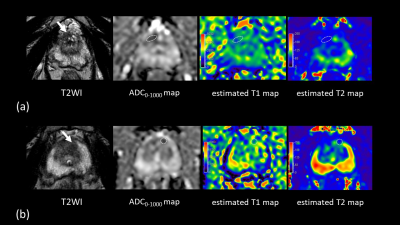
Biopsy-proven prostate cancers and noncancers among PI-RADS category 3 lesions were identified in 13 lesions and 15 lesions, respectively. Estimated T1 and T2 in PI-RADS category 3 target biopsy-proven cancers were significantly lower (1969 ms [1837, 2091] and 59 ms [55, 63], respectively) than noncancers (2101 ms [2024, 2219] and 75 ms [63, 88], respectively) (P = 0.04 for T1 and P= 0.01 for T2), while there is no significant difference in ADC0-1000 between cancers and noncancers (1.01 mm2/s [0.88, 1.25] and 1.19 mm2/s [1.00, 1.32], respectively; P = 0.28) in Figure 3. Data are presented as medians [25th and 75th percentiles]. For separation between PI-RADS category 3 cancers and noncancers, the AUC for T2 (0.79) was higher than the AUC for T1 (0.72) and ADC0-1000 (0.62) in Figure 4. Cut-off value was 74 ms for estimated T2, 2010 ms for estimated T1, and 1.07 mm2/s for ADC0-1000, respectively. The combination of estimated T1, T2, and ADC0-1000 yielded an AUC of 0.85. Figure 5 shows representative clinical cases with cancer and noncancer. Some studies have shown that the volume fractions of epithelium, stroma, and lumen space change because of PCa14, 15. Therefore, estimated T1 and T2 may be used to capture microstructural changes, especially the difference in lumen space between cancer and noncancer that did not show differences in ADC. With this approach, in addition to the conventional DW images at b-value of 1000 and 2000, ADC map, and estimated T1 and T2 map, synthetization of DW image for each b-value can be acquired in approximately 4 minutes, which is acceptable scan time in clinical practice.Conclusion
Our results indicate that estimated T1 and T2 calculated from MRME-DWI acquisition used for synthetization of DW images might enable more accurate diagnosis of suspicious lesions with PI-RADS category 3. These quantitative values could have the potential to reduce unnecessary biopsies.Acknowledgements
No acknowledgement found.References
1. Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 2019; 76: 340-51.
2. Xu L, Zhang G, Zhang D, et al. Comparison of PI-RADS version 2.1 and PI-RADS version 2 regarding interreader variability and diagnostic accuracy for transition zone prostate cancer. Abdom Radiol 2020; 45: 4133-41.
3. Kido A, Tamada T, Kanomata N, et al. Multidimensional analysis of clinicopathological characteristics of false-negative clinically significant prostate cancers on multiparametric MRI of the prostate in Japanese men. Jpn J Radiol 2019; 37: 154-64.
4. Borofsky S, George AK, Gaur S, et al. What Are We Missing? False-Negative Cancers at Multiparametric MR Imaging of the Prostate. Radiology 2018; 286: 186-95.
5. Woo S, Suh CH, Kim SY, et al. Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol 2017; 72: 177-88.
6. Tamada T, Kido A, Yamamoto A, et al. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2.1. J Magn Reson Imaging 2021; 53: 283-291.
7. Stolk TT, de Jong IJ, Kwee TC, et al. False positives in PIRADS (V2) 3, 4, and 5 lesions: relationship with reader experience and zonal location. Abdom Radiol (NY) 2019; 44: 1044-51.
8. Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for clinical neuroimaging: Results of the magnetic resonance image compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am J Neuroradiol 2017; 38: 1103-10.
9. Hagiwara A, Hori M, Yokoyama K, et al. Synthetic MRI in the detection of multiple sclerosis plaques. AJNR Am J Neuroradiol 2017; 38: 257-63.
10. Ueda Y, Tamada T, Sanai H, et al. Feasibility of synthetic short TR and short TE DWI in prostate cancer. Proceedings of the 30th Annual Meeting of ISMRM, 2022 #3711.
11. Yu AC, Badve C, Ponsky LE, Pahwa S, et al. Development of a Combined MR Fingerprinting and Diffusion Examination for Prostate Cancer. Radiology 2017; 283: 729-38.
12. Shiradkar R, Panda A, Leo P, et al. T1 and T2 MR fingerprinting measurements of prostate cancer and prostatitis correlate with deep learning-derived estimates of epithelium, lumen, and stromal composition on corresponding whole mount histopathology. Eur Radiol 2021;31: 1336-46.
13. Panda A, Obmann VC, Lo WC, et al. MR Fingerprinting and ADC Mapping for Characterization of Lesions in the Transition Zone of the Prostate Gland. Radiology 2019; 292: 685-94.
14. Langer DL, van der Kwast TH, Evans AJ, et al. Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 2010; 255: 485-94.
15. Chatterjee A, Watson G, Myint E, et al. Changes in epithelium, stroma, and lumen space correlate more strongly with gleason pattern and are stronger predictors of prostate ADC changes than cellularity metrics. Radiology 2015; 277: 751-62.
Figures