1862
Sub-differentiation of PI-RADS 3 lesions in TZ by advanced diffusion-weighted imaging to aid the biopsy decision process
Kun-Peng Zhou1, Jie Bian2, Hua-Bin Huang1, Chao Bu1, and Li-Zhi Xie3
1Radiology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China, 2Radiology, the second hospital of dalian medical university, dalian, China, 3GE Healthcare, Beijing, China, Beijing, China
1Radiology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China, 2Radiology, the second hospital of dalian medical university, dalian, China, 3GE Healthcare, Beijing, China, Beijing, China
Synopsis
Keywords: Prostate, Prostate
The aim of this study was to investigate the use of advanced diffusion-weighted imaging (IVIM, stretched exponential model and DKI) to sub-differentiation of TZ PI-RADS 3 lesions and aid the biopsy decision process. Finally, the logistic model could correctly classify 89.39% of the subjects. The results of ROC analysis showed that AUC was 0.9197, CI 95%: 0.8736-0.9659. Sensitivity, specificity, positive predictive value and negative predictive value were 92.1%, 80.4%, 93.9% and 75.5%, respectively. Therefore, advanced diffusion-weighted imaging can predict PCa in TZ PI-RADS 3 lesions and inform the decision-making process of whether or not to perform a biopsy.Introduction
Prostate Imaging-Reporting and Data System (PI-RADS) has been generally accepted (1). Performing a biopsy for these PI-RADS 3 lesions (intermediate lesions) is still a matter of debate(1, 2). For PI-RADS 3 lesions, several studies have found that about 6.5%-60% were confirmed as prostate cancer (PCa) by biopsy, while 4.1%-21% were clinically significant prostate cancer (csPCa, Gleason≥3+4)(2-4). Gosein et al(5) reported that 27.3% of PI-RADS 3 lesions located in transition zone (TZ) were PCa, among which 9.1% were csPCa. Several studies have shown that PCa in PI-RADS 3 have higher PSA density, lower prostate volume, and apparent diffusion coefficient (ADC) value compared with benign lesions in PI-RADS 3(6-9). Using conventional scanning sequences makes it difficult to distinguish TZ cancers from fibromuscular (stromal) benign prostatic hyperplasia (BPH) as both of these conditions can manifest low T2 signal intensity on MR images. some studies have demonstrated that advanced diffusion-weighted imaging was useful in the detection and assessment of PCa aggressiveness(10-12). The purpose of this study is sub-differentiation of TZ PI-RADS 3 lesions using intravoxel incoherent motion (IVIM), stretched exponential model, and diffusion kurtosis imaging (DKI) to aid the biopsy decision process.Methods
Our study was approved by the institutional review board and all patients signed informed consent at screening. 637 patients underwent prostate MRI examination at 3.0 T MRI (GE Discovery MR 750W) with 8-channel phased array coil, including conventional scanning sequences, IVIM, stretched exponential model and DKI. According to PI-RADS Version 2.1, our study including 145 patients with 198 PI-RADS assessment category 3 lesions located in TZ with pathologic data which obtained by Magnetic resonance imaging-Transrectal ultrasound (MRI-TRUS) fusion biopsy. Flinally, 149 lesions were BPH and 49 lesions were PCa. Acquisition of quantitative data was carried on GE AW 4.6 workstation. ROI was then drawn on the parameter maps of IVIM, stretched exponential model and DKI. Three ROIs with area of 50 mm2 were randomly drawn at the maximum slicer of the lesion, and the average value was recorded. In order to examine the association between parameters of DKI, IVIM, and stretched exponential model with the probability of PCa in PI-RADS 3 lesions, binary logistic regression analysis was applied. After binary logistic regression analysis, the receiver operating characteristic (ROC) curve of combination parameters, which were statistically significant in binary logistic regression analysis, were analyzed to test the diagnostic efficiency of detecting PCa in PI-RADS 3 lesions.Results
Among 198 PI-RADS category 3 lesions, 75.25% (149/198) lesions were pathologically confirmed as BPH, and 24.75% (49/198) lesions were PCa. Of the 145 patients with PI-RADS category 3 lesions, 68.28% (99/145) were BPH and 31.72% (46/145) were PCa (Figure 1-2). Finally, the logistic model was statistically significant, χ2=181.410, p<0.001. The model could correctly classify 89.39% of the subjects. Among the nine independent variables included in the model, only FA (p=0.004), MD (p=0.005), MK (p=0.015), D (p=0.001), DDC (p=0.038) were statistically significant. Odds ratio (CI 95%) were 3.165 (2.169-8.645), 0.158 (0.008-0.427), 2.886 (1.365-7.491), 0.185 (0.019-0.382), 0.221 (0.053-0.917), respectively (Figure 3). The results of ROC analysis showed that area under the curve (AUC) was 0.9197, CI 95%: 0.8736-0.9659. Sensitivity, specificity, positive predictive value and negative predictive value were 92.1%, 80.4%, 93.9% and 75.5%, respectively.Discussion
This study built a multimodal prostate MRI to distinguish PCa from PI-RADS 3 lesions. We found that combination of parameters FA, MD and MK of DKI, parameter D of IVIM and parameter DDC of stretched exponential model has high diagnostic efficacy for PCa in PI-RADS 3 lesions. This may be explained by the fact that water in PCa meet non-Gaussian diffusion. What’s more, due to the small volume and compact arrangement of tumor cells, leading to the diffusion restriction of water molecules increased. However, unlike some previous studies, our results showed that apparent diffusion coefficient (ADC) has no statistical significance in our modal. This may be related to ADC not only contained pure diffusion, but also affected by perfusion. Parameters pseudodiffusion coefficient (D*) and perfusion fraction (f) of IVIM, parameter heterogeneity index (α) of stretched exponential model also have no statistical significance. A reasonable explanation may be that the perfusion difference between PCa and BPH may not be enough to be detected by D* and f in TZ. In addition, necrosis rarely occurred in PCa, resulting in low heterogeneity of the tumor.Conclusion
This preliminary study shows that parameters (FA, MD, MK, D, and DDC) of advanced diffusion-weighted imaging (IVIM, stretched exponential model and DKI) can predict PCa in TZ PI-RADS 3 lesions and inform the decision-making process of whether or not to perform a biopsy.Acknowledgements
No acknowledgement found.References
1. Hansen NL, Barrett T, Koo B, Doble A, Gnanapragasam V, Warren A, et al. The influence of prostate-specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7-10 prostate cancer in a repeat biopsy setting. BJU Int. 2017;119(5):724-30.2. Hansen N, Patruno G, Wadhwa K, Gaziev G, Miano R, Barrett T, et al. Magnetic Resonance and Ultrasound Image Fusion Supported Transperineal Prostate Biopsy Using the Ginsburg Protocol: Technique, Learning Points, and Biopsy Results. Eur Urol. 2016;70(2):332-40.3. Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-22.4. Rosenkrantz AB, Meng X, Ream JM, Babb JS, Deng FM, Rusinek H, et al. Likert score 3 prostate lesions: Association between whole-lesion ADC metrics and pathologic findings at MRI/ultrasound fusion targeted biopsy. J Magn Reson Imaging. 2016;43(2):325-32.5. Gosein M, Pang E, Chang S, Black P, Goldenberg L, Harris A, et al. Outcomes of Magnetic Resonance Imaging-Ultrasound Fusion Prostate Biopsy of PI-RADS 3, 4, and 5 Lesions. Can Assoc Radiol J. 2018;69(3):303-10.6. Kim M, Ryu H, Lee HJ, Hwang SI, Choe G, Hong SK. Who can safely evade a magnetic resonance imaging fusion-targeted biopsy (MRIFTB) for prostate imaging reporting and data system (PI-RADS) 3 lesion? World J Urol. 2021;39(5):1463-71.7. Gortz M, Radtke JP, Hatiboglu G, Schutz V, Tosev G, Guttlein M, et al. The Value of Prostate-specific Antigen Density for Prostate Imaging-Reporting and Data System 3 Lesions on Multiparametric Magnetic Resonance Imaging: A Strategy to Avoid Unnecessary Prostate Biopsies. Eur Urol Focus. 2021;7(2):325-31.8. Hermie I, Van Besien J, De Visschere P, Lumen N, Decaestecker K. Which clinical and radiological characteristics can predict clinically significant prostate cancer in PI-RADS 3 lesions? A retrospective study in a high-volume academic center. Eur J Radiol. 2019;114:92-8.9. Al Hussein Al Awamlh B, Marks LS, Sonn GA, Natarajan S, Fan RE, Gross MD, et al. Multicenter analysis of clinical and MRI characteristics associated with detecting clinically significant prostate cancer in PI-RADS (v2.0) category 3 lesions. Urol Oncol. 2020;38(7):637 e9- e15.10. Valerio M, Zini C, Fierro D, Giura F, Colarieti A, Giuliani A, et al. 3T multiparametric MRI of the prostate: Does intravoxel incoherent motion diffusion imaging have a role in the detection and stratification of prostate cancer in the peripheral zone? Eur J Radiol. 2016;85(4):790-4.11. Wang X, Tu N, Qin T, Xing F, Wang P, Wu G. Diffusion Kurtosis Imaging Combined With DWI at 3-T MRI for Detection and Assessment of Aggressiveness of Prostate Cancer. AJR Am J Roentgenol. 2018;211(4):797-804.12. Shan Y, Chen X, Liu K, Zeng M, Zhou J. Prostate cancer aggressive prediction: preponderant diagnostic performances of intravoxel incoherent motion (IVIM) imaging and diffusion kurtosis imaging (DKI) beyond ADC at 3.0 T scanner with gleason score at final pathology. Abdom Radiol (NY). 2019;44(10):3441-52.Figures
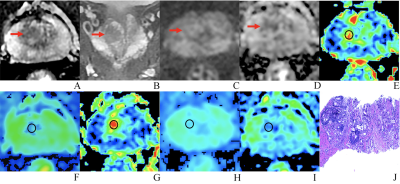
A 76-year-old man with BPH and PSA of 4.48 ng/ml. (A-D) The lesion with PI-RADS 3 seen in the right middle transition zone. (E) Fractional anisotropy (FA) of 0.201. (F) Mean diffusion (MD) of 1.214×10-3mm2/s. (G) Mean kurtosis (MK) of 0.512. (H) Diffusion coefficient (D) of 0.795×10-3mm2/s. (I) Distribute diffusion coefficient (DDC) of 0.942×10-3mm2/s. (J) HE staining, ×100.
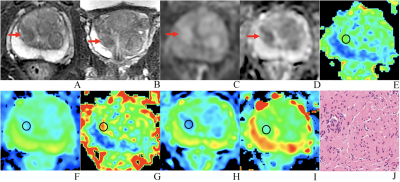
An 81 years old man with PCa, PSA of 6.47 ng/ml, and Gleason 3+3. (A-D) The lesion with PI-RADS 3 seen in the right middle transition zone. (E) Fractional anisotropy (FA) of 0.257. (F) Mean diffusion (MD) of 1.018×10-3mm2/s. (G) Mean kurtosis (MK) of 0.754. (H) Diffusion coefficient (D) of 0.588×10-3mm2/s. (I) Distribute diffusion coefficient (DDC) of 0.717×10-3mm2/s. (J) HE staining, ×100.
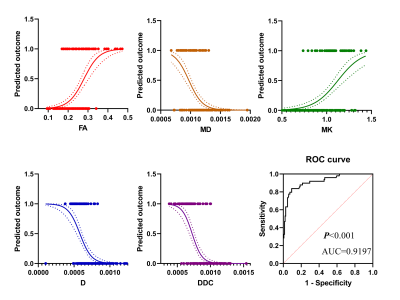
The results of binary logistic regression analysis showed that in pi-rads 3 lesions, the prevalence of PCa increased with the increase of FA value and MK value but decreased with the increase of MD value, D value, and DDC value. The diagnostic efficiency in distinguishing PCa from PI-RADS 3 lesions of these parameters was 0.9197.
DOI: https://doi.org/10.58530/2023/1862