1859
Prognostic Value of ADC Histogram Analysis in Men with High Risk Prostate Cancer Receiving Adjuvant Hormonal Therapy after Radical Prostatectomy1Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Synopsis
Keywords: Prostate, Cancer, high-risk prostate cancer, biochemical recurrence
直方图分析是医学图像的定量后处理方法,被认为在癌症管理中很有价值。我们使用表观扩散系数 (ADC) 图的直方图参数分析了在根治性前列腺切除术 (RP) 后接受辅助激素治疗 (AHT) 的高危前列腺癌 (PCa) 患者的结果。我们观察到,较低的 ADC 50 和较高的峰度可以独立预测较差的生化无复发生存期 (BCR-fs),并且当添加直方图参数时,预后模型的性能会有所提高。我们的研究结果显示了 ADC 直方图分析在这一特定的 PCa 患者亚组中的增量预后价值。INTRODUCTION
Prostate cancer (PCa) is the second most common cancer and the fifth leading cause of cancer-related deaths globally1. Individuals with high-risk PCa may benefit from multimodal therapy strategies, such as a reasonable first step of radical prostatectomy (RP) to reduce the tumor burden and following adjuvant hormonal therapy (AHT)2. A subset of high-risk PCa patients, however, may exhibit early resistance against AHT and elevated PSA levels in the short term after RP, which has led to studies of prognostic factors. The apparent diffusion coefficient (ADC), derived from diffusion-weighted(DW) MRI, holds promise as a reliable prognostic biomarker3,4. We aimed to investigate the incremental prognostic value of ADC devired parameters for PCa patients who underwent RP and AHT, using a quantitative method called histogram analysis of the ADC map.METHODS
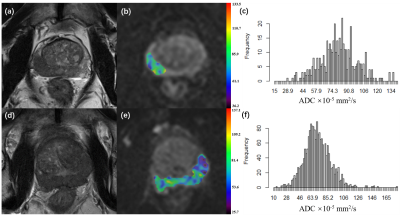
Sixty-two high-risk PCa patients (preoperative serum PSA ≥ 20 ng/mL, or Gleason score ≥ 8, or pT3/4, N0M0 or any T, N1M0, or positive surgical margins), who received immediate administration with AHT after surgery (medical castration (LHRHa), combined with anti-androgens (bicalutamide, etc.)), were enrolled. Prostate MRI examinations were preoperatively performed with a 3.0T scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) on all patients. A 3D volume of interest of PCa foci was manually delineated on DW-images with a custom-developed software (Firevoxel, https://firevoxel.org). The ADC map was calculated by DW-images at b = 0 and 1000 s/mm2, and the ADC histogram was automatically generated along with the following corresponding parameters: mean ADC (ADCmean), median ADC (ADC50), 10th percentile ADC (ADC10), 90th percentile ADC (ADC90), skewness, and kurtosis. The survival outcome was biochemical recurrence (BCR), which was defined as a PSA increase for two consecutive measurements, and PSA level ≥ 0.2 ng/ml for localized disease and an increase in the PSA level by 25% or more above the nadir (and by ≥2ng/ ml), with confirmation four or more weeks later for lymphatic or distant metastasis, according to PCWG3 criteria5. Survival analysis was performed with the Kaplan-Meier method and Cox regression analysis. Prognostic models were built in two ways: one clinical model without ADC parameters, and the other clinical-imaging model with ADC parameters as covariates, the performances of which were compared to assess the incremental prognostic value of the ADC parameters. The statistics analysis was performed using R (Version 4.1.2) and Stata (version 12.1).RESULTS
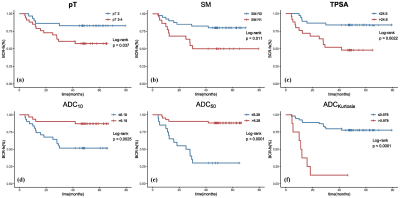
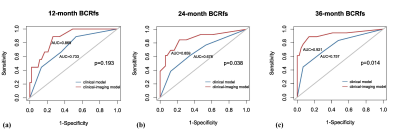
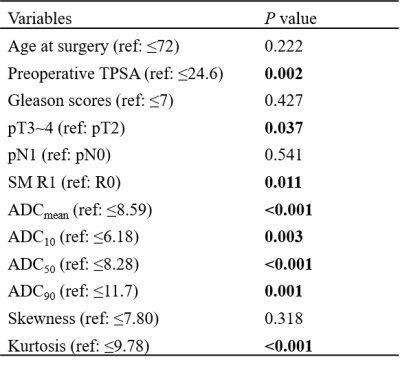
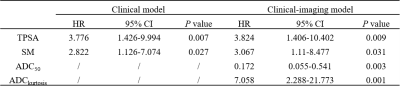
All patients were followed up for more than 3 years with a median follow-up period of 54.1 months (range, 41.1–79.3 months). BCR was observed in 19 (30.6%) patients. All ADC percentile values, kurtosis, SM, TPSA, and pT were significantly associated with BCR-free survival (BRC-fs) in the univariate survival analysis. ADC50 and kurtosis remained significant after adjustment for clinical factors. The clinical model was built on TPSA and SM, while the clinical-imaging models were built with ADC50 and kurtosis as the covariables. The Harrell concordance index of clinical-imaging model was higher than that of clinical model. The diagnostic accuracy of models for the 24- and 36-month BCR-fs increased significantly when histogram parameters were added by comparing the area under the receiver operating characteristic curves (AUC).DISCUSSION
Identifying patients at risk of developing BCR is of clinical significance, especially for high-risk PCa. We evaluated the value of ADC histogram derived from preoperative DWI in predicting the BCR of a specific sub-group of patients: high-risk PCa patients who underwent RP followed by AHT. The result showed that ADC50 and kurtosis were independent prognosis factors. However, a previous study found that ADC0-10 was better than the others to predict the event of BCR6, which was explained that the lower percentiles like ADC10 were more representative of focal areas of high cellularity so were more associated with the prognosis. Inconsistent results may be attributed to different study populations, because we enrolled patients with high-risk PCa, whose cancer foci may be more prone to necrosis and hemorrhage. Such micro-necrosis and micro-hemorrhage could increase ADC values but also have prognostic significance, thus higher percentile ADCs may more comprehensively predict the outcome. In addition, ADC kurtosis, which is an important index representing the degree of homogeneity of the tumors, has been reported to be associated with the outcome of several cancers7,8. Our result was consistent with previous studies that high kurtosis represented high aggressiveness and poor prognosis.Finally, we observed that ADC histogram analysis held incremental prognostic value by comparing the two prognostic models. As a result, this quantitative analysis method was promising to be integrated into clinical tools for more accurate prediction of BCR.CONCLUSION
ADC histogram analysis derived from preoperative MRI has incremental prognostic value as an imaging biomarker for the prognosis of patients with high-risk PCa who received AHT after RP. Our study results demonstrate that ADC50 and kurtosis are the most valuable histogram-derived parameters for the prediction of BCR-fs in patients with high-risk PCa. Future validation in independent cohorts remains warranted to provide stronger evidence for the use of histogram analysis in clinical practice.Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant numbers 82071889 and 62131009).The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.References
1.Sung H, Ferlay J, Siegel RL et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209-49.
2.Mottet N, Bellmunt J, Bolla M et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017; 71: 618-29.
3.Prostate cancer: role of pretreatment multiparametric 3-T MRI in predicting biochemical recurrence after radical prostatectomy. AJR American journal of roentgenology 2014; 202: W459-65.
4.Hattori S, Kosaka T, Mizuno R et al. Prognostic value of preoperative multiparametric magnetic resonance imaging (MRI) for predicting biochemical recurrence after radical prostatectomy. BJU Int 2014; 113: 741-7.
5.Scher HI, Morris MJ, Stadler WM et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016; 34: 1402-18.
6.Rosenkrantz AB, Ream JM, Nolan P et al. Prostate Cancer: Utility of Whole-Lesion Apparent Diffusion Coefficient Metrics for Prediction of Biochemical Recurrence After Radical Prostatectomy. AJR American journal of roentgenology 2015; 205: 1208-14.
7.Takahashi Y, Hayano K, Ohira G et al. Histogram Analysis of Diffusion-Weighted MR Imaging as a Biomarker to Predict Survival of Surgically Treated Colorectal Cancer Patients. Dig Dis Sci 2021; 66: 1227-32.
8.Hirata A, Hayano K, Ohira G et al. Volumetric Histogram Analysis of Apparent Diffusion Coefficient as a Biomarker to Predict Survival of Esophageal Cancer Patients. Ann Surg Oncol 2020; 27: 3083-9.
Figures