1858
ADC as a quantitative value: classifying lymph node dignity in prostate cancer and inter-scanner variability1Department of Clinical Radiology, University of Muenster, Münster, Germany, 2Institute of Biostatistics and Clinical Research, University of Muenster, Muenster, Germany
Synopsis
Keywords: Prostate, Diffusion/other diffusion imaging techniques, lymph node, reproducibility
Apparent diffusion coefficient (ADC) has potential as a quantitative imaging biomarker for differentiation of malignant from benign tissue. Here, we present a phantom and in vivo measurement of ADC reproducibility and a restrospective study in over 100 patients with prostate cancer to evaluate ADC as a classifier.Introduction
Reliable classification of lymph node dignity could be a prerequisite for individual therapy in prostate cancer. Several studies indicated significant potential of the apparent diffusion coefficient (ADC) as a quantitative imaging biomarker for differentiation of malignant from benign tissue [1,2]. However, previous studies on the suitability of the ADC as a classifier for lymph nodes in prostate cancer are rather heterogeneous [3-8 ]. Additionally, significant inter-scanner variability has been found for quantitative measurements of the ADC [9-11]. Lack of guidelines and standardized protocols further hampers transferability between vendors.Therefore, the objective of this work was to evaluate the ADC reproducibility with regard to coil configuration and vendor specific properties using both phantom and in-vivo measurements. In a second step the suitability of the ADC for classification of benign and malignant lymph nodes was assessed using clinical PSMA-PET/MRI scans including DWI.
Methods
All DWI measurements were done on a 3T PET/MRI (Biograph mMR, Siemens Healthineers) and a 3T MRI (Philips MR7700) system using clinical protocols and coils. Table 1 shows the different sequence parameters. .| Scanner | Sequ. | Accel. | TE [ms] | TR [ms] | Slices /stack [mm] | SliceGap [mm] | Voxelsize (aqu.) [mm] | FoV [mm] | b-values [s/mm²] | Fat-suppr. |
| PET/MR | SE-EPI | Grappa 2 | 86 | 6400 | 21 | 1 | 2*2*5 | 380 * 285 | 0/50/400/800 | SPAIR |
| MR7700 | SE-EPI | no | 84 | 2200 | 21 | 0.5 | 2*2*5 | 380 * 285 | 0/50/400/800 | SPIR |
All phantom measurements were performed using a commercially available DWI phantom (CaliberMRI Diffusion Phantom) with MR visible thermometer to adjust for temperature effect. The phantom consists of 13 tubes. Two each with concentrations of 10%, 20%, 30%, 40% and 50% and three tubes with 0% polyvinylpyrrolidone (PVP) resulting in a range of target ADC values from 293 *10-6 mm²/s to 2106 * 10-6 mm²/s. ADCmean measurements were repeated 5 times under equal conditions with each scanner.
13 patients (mean age 68.0 ± 6.0 years) with prostate cancer underwent MRI on both scanners. Both times the respective clinical routine protocol as well as the respective coil configuration were used (see Table 1). ADC maps were calculated on each system and evaluated on the in-house PACS-viewer (General Electric Centricity PACS RA1000). ADCmean measurements were made on 86 lymph nodes identified on T1 weighted images.
Additionally, 101 patients (mean age 68.5 ± 8.1 years) were examined using PSMA-PET/MRI (including DWI). 359 retroperitoneal and pelvic lymph nodes suitable for ADC measurement were identified (143 metastatic with focal tracer signal, 216 benign without tracer signal).
Results
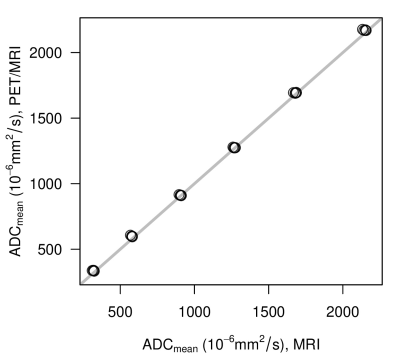
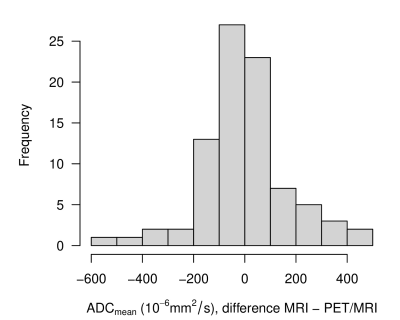
ADC of phantom measurements on the different scanners were highly comparable (R²>0.99) over the entire range of PVP concentrations (Figure 1).Also, the ADC values from the in-vivo measurements revealed only small scanner bias (mean difference 2.14x10-6 mm²/s). Interclass correlation coefficient (ICC) was calculated with 0.893, 95% CI [0.840, 0.930]. Figure 2 shows the frequency distribution of deviations per lymph node.
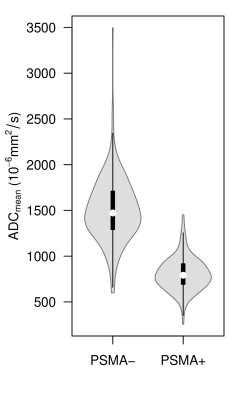
PSMA+ lymph nodes showed significantly restricted diffusion compared to those with no PSMA-signal (769 * 10-6 mm²/s vs. 1463 * 10-6 mm²/s, p < 0.0001). Figure 3 shows the distribution of ADCmean for both groups of lymph nodes (PSMA- vs. PSMA+).
Discussion
In order to be able to use the ADC as a quantitative diagnostic marker of lymph node dignity, certain prerequisites must be met: First the difference in the ADC between malign and benign lymph nodes must be sufficiently large and second - to generalize these results - robustness and independence from the manufacturer and coils must be ensured.Our results from 101 patients investigated with PET/MR show that benign and malign lymph nodes differ significantly concerning the ADC corroborating previous studies [4,6].
A good transferability between our MR systems can be assumed due to the good agreement between phantom measurements and in-vivo measurements. There are smaller deviations and bias between MRI systems, but ultimately these are negligible compared to the above-mentioned differences in ADC between benign and malign lymph nodes.
In order to generalize these findings, however, further investigations are necessary: The position of the lymph nodes relative to the isocenter should be examined. The influence of various sequence parameters on the ADC must also be checked and finally a comparison should be made at different field strengths.
Acknowledgements
No acknowledgement found.References
[1] Baliyan V, Das CJ, Sharma R, Gupta AK (2016) Diffusion weighted imaging: Technique and applications. World J Radiol 8(9):785–98.
[2] White NS, McDonald C, McDonald CR et al. (2014) Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res 74(17):4638–52.
[3] Eiber M, Beer AJ, Holzapfel K et al. (2010) Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest Radiol 45(1):15–23.
[4] Beer AJ, Eiber M, Souvatzoglou M et al. (2011) Restricted water diffusibility as measured by diffusion-weighted MR imaging and choline uptake in (11)C-choline PET/CT are correlated in pelvic lymph nodes in patients with prostate cancer. Mol Imaging Biol 13(2):352–61.
[5] Vag T, Heck MM, Beer AJ et al. (2014) Preoperative lymph node staging in patients with primary prostate cancer: comparison and correlation of quantitative imaging parameters in diffusion-weighted imaging and 11C-choline PET/CT. Eur Radiol 24(8):1821–6.
[6] Vallini V, Ortori S, Boraschi P et al. (2016) Staging of pelvic lymph nodes in patients with prostate cancer: Usefulness of multiple b value SE-EPI diffusion-weighted imaging on a 3.0 T MR system. Eur J Radiol Open 3:16–21.
[7] Roy C, Bierry G, Matau A, Bazille G, Pasquali R (2010) Value of diffusion-weighted imaging to detect small malignant pelvic lymph nodes at 3 T. Eur Radiol 20(8):1803–11.
[8] Thoeny HC, Froehlich JM, Triantafyllou M et al. (2014) Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted MR imaging. Radiology 273(1):125–35.
[9] Ye, X. H., Gao, J. Y., Yang, Z. H., & Liu, Y. (2014). Apparent diffusion coefficient reproducibility of the pancreas measured at different MR scanners using diffusion‐weighted imaging. Journal of Magnetic Resonance Imaging, 40(6), 1375-1381.
[10] Michoux, N. F., Ceranka, J. W., Vandemeulebroucke, J., Peeters, F., Lu, P., Absil, J., ... & Lecouvet, F. E. (2021). Repeatability and reproducibility of ADC measurements: a prospective multicenter whole-body-MRI study. European radiology, 31(7), 4514-4527.
[11] Hoang-Dinh, A., Nguyen-Quang, T., Bui-Van, L., Gonindard-Melodelima, C., Souchon, R., & Rouvière, O. (2022). Reproducibility of apparent diffusion coefficient measurement in normal prostate peripheral zone at 1.5 T MRI. Diagnostic and Interventional Imaging.