1848
Fast Free-Breathing 3D T1ρ Abdominal Imaging Using an Efficient Diamond Radial Sampling Strategy at 3T1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Department of Radiology, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY, United States, 3MR Clinical Science, Philips Healthcare, Cambridge, MA, United States, 4Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Keywords: Liver, Relaxometry
An efficient diamond radial sampling strategy was proposed for free-breathing 3D T1ρ abdominal imaging at 3T. The phantom experiment shows that diamond radial sampling provides T1ρ measurement values comparable to that of 3D Cartesian and radial stack-of-stars sampling. In-vivo volunteer studies illustrate that diamond radial sampling is superior to Cartesian sampling, where image quality is significantly compromised by breathing motion artifacts. In addition, scan time can be drastically reduced using the fast MAPSS method. This work demonstrates the feasibility of quantitative free-breathing 3D T1ρ imaging with diamond radial sampling in the abdomen.INTRODUCTION
T1ρ imaging has been mostly employed to studying cartilage due to its high sensitivity to low-frequency interactions between water molecules and their local macromolecular environment [1]. The application of T1ρ imaging in the abdomen is more challenging due to respiratory motion, field inhomogeneities within a large field of view, and unavailability of localized transmit/receive coils. Most of the previous work on abdominal T1ρ imaging were performed under breath-holds [2-4], which leads to low spatial resolution and limited volumetric coverage. Radial sampling is inherently less sensitive to respiratory motion compared to Cartesian sampling and 3D radial stack-of-stars sampling was recently used to acquire data for each golden angle after T1ρ preparation in human liver [5]. However, data acquisition is slow (4:41 min for each TSL). The aim of this work was to develop a fast free-breathing 3D abdominal T1ρ imaging technique using efficient diamond radial sampling in combination with the state-of-the-art magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (MAPSS) sequence [6].METHODS
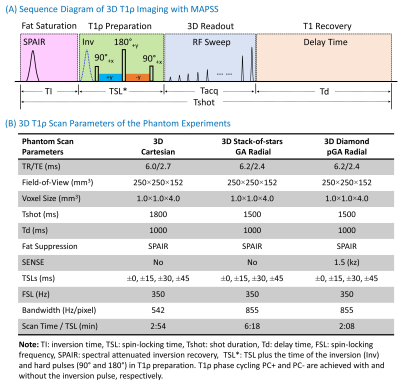
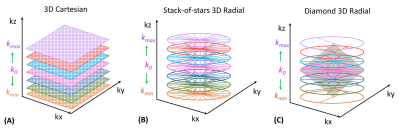
3D T1ρ Sequence: The 3D T1ρ sequence consists of four components (Figure 1A): fat suppression (SPAIR), T1ρ preparation, 3D readout, and T1 recovery. RF pulse flip angle sweeping and T1ρ phase cycling (with and without an adiabatic inversion pulse) are used for 3D MAPSS [7]. Three different k-space trajectories are used for 3D readout (Figure 2) with low-high profile ordering: Cartesian, stack-of-stars golden-angle radial (SOS-GAR), and diamond pseudo-golden-angle radial (D-pGAR). The sampling density along kz is constant for SOS-GAR but is decreased from the center (k0) to the peripheral k-space (kmax, kmin) for D-pGAR.Phantom Study: The 3D T1ρ sequences were first tested on a phantom which consists of three pairs of tubes with three different agarose concentrations (2%, 3%, and 4%) embedded in a cylindrical plastic holder (The Phantom Laboratory, Greenwich, NY). The phantom scan parameters are shown in Figure 1B.
Volunteer Study: Free-breathing 3D T1ρ abdominal imaging was performed on a healthy volunteer (male, 35 years old) with Cartesian and D-pGAR sampling. SOS-GAR was not performed due to the prohibitively long scan time. The volunteer scan parameters were similar to the phantom experiments, except the following: FOV=360×360×200 mm3, voxel size = 1.5×1.5×4.0 mm3, TR/TE = 5.6/2.5 ms for Cartesian and 5.6/2.2 ms for D-pGAR, SENSE = 1.4×1.8 (ky×kz) for Cartesian and 1.5 (kz) for D-pGAR, scan time per TSL = 0:52 min for Cartesian and 2:26 min for D-pGAR. All experiments were performed on a clinical 3T MRI scanner (Ingenia Elition X, Philips Healthcare) using anterior and posterior coils.
T1ρ Map and Analysis: T1ρ maps were generated using an in-house software in Interactive Data Language (ExelisVis, Boulder, CO) and the fitting methods for traditional MAPSS (8 TSLs) and fast MAPSS (4 TSLs or 3 TSLs) were reported previously [8]. Interleaved phase cycling starting with a positive phase (e.g., +0, -15, +30, -45 ms) was used for the fast MAPSS methods. Five ROIs (liver, pancreas, spleen, anterior muscle, and posterior muscle) were manually drawn on a selected slice to evaluate the T1ρ values of these anatomical structures in the volunteer.
RESULTS
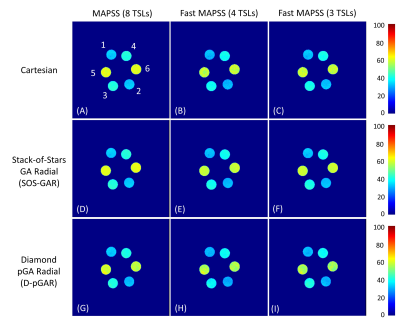
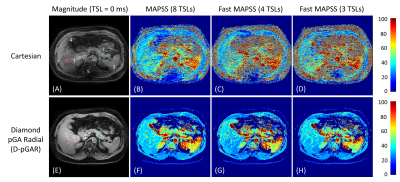
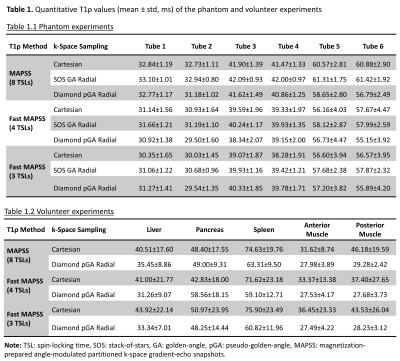
Quantitative T1ρ maps of the phantom are shown in Figure 3. The T1ρ maps generated from SOS-GAR and D-pGAR present high concordance to the ones using Cartesian sampling. The fast MAPSS methods (4 TSLs and 3 TSLs) show similar T1ρ maps compared to the reference MAPSS method with 8 TSLs. Table 1.1 summarizes the quantitative T1ρ values of the six tubes in the phantom experiments. The results further confirm that the T1ρ measurements were comparable among the three sampling methods. Figure 4 illustrates the magnitude image (TSL = 0 ms) and the corresponding T1ρ maps of the volunteer. The image quality and T1ρ maps of Cartesian sampling was significantly degraded by breathing motion artifacts, which was drastically improved using D-pGAR sampling. The T1ρ values of the five ROIs are shown in Table 1.2.DISCUSSION
Free-breathing abdominal 3D T1ρ imaging with Cartesian sampling is significantly compromised by breathing motion artifacts if no triggering or gating method is used. This work demonstrated that the proposed efficient diamond radial sampling approach (D-pGAR) significantly improved image quality and T1ρ quantification compared to Cartesian sampling. The phantom experiment demonstrated that T1ρ measurements from D-pGAR sampling were comparable to that of the traditional Cartesian and SOS-GAR sampling methods. This work further supports the use of fast MAPSS with only 4 TSLs or 3 TSLs, which provides similar T1ρ results compared to the reference MAPSS with 8 TSLs [8]. Fast MAPSS sequences can significantly reduce scan time for T1rho mapping (i.e., the total scan time is reduced from 19 minutes to 7 minutes if only 3 TSLs are used). Although the use of D-pGAR sampling was demonstrated for 3D T1ρ imaging, it can be similarly used for other quantitative parameter mapping with a different magnetization preparation module, such as T2 mapping, chemical exchange saturation transfer imaging, and magnetization transfer imaging.CONCLUSION
Free-breathing 3D T1ρ abdominal imaging with clinically feasible scan times was demonstrated with the use of an efficient diamond radial sampling strategy. The combination of diamond radial sampling and fast MAPSS acquisition would enable efficient quantitative evaluation of tissue properties in abdominal organs.Acknowledgements
The work was supported by NIH Grant R01-AR076328.References
1. Li X, Ma B, Link TM, Castillo D, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T1rho and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 Tesla MRI. Osteoarthritis Cartilage 2007; 15(7): 789-797.
2. Chen W, Chan Q, Wang Y. Breath-hold black blood quantitative T1rho imaging of liver using single shot fast spin echo acquisition. Quant Imaging Med Surg 2016; 6(2): 168-177.
3. Wang Y, Deng M, Lin J, Kwok, Liu E, Chen W. Age- and gender-associated liver physiological T1rho dynamics demonstrated with a clinically applicable single-breathold acquisition. SLAS Technol 2018; 23(2): 179-187.
4. Wang Y, Deng M, Lo GG, Liang D, Yuan J, Chen W. Breath-hold black-blood T1rho mapping improves liver T1rho quantification in healthy volunteers. Acta Radiol 2018; 59(3): 257-265.
5. Sharafi A, Baboli R, Zibetti M, Shanbhogue K, Olsen S, Block T, Chandarana H, Regatte R. Volumetric multicomponent T1ρ relaxation mapping of the human liver under free breathing at 3T. Magn Reson Med 2020; 83(6):2042-2050.
6. Li X, Han ET, Busse RF, Majumdar S. In vivo T1ρ mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med 2008; 59(2): 298-307.
7. Peng G, Wu C, Peng Q. Improved phase-cycling preparations in quantitative T1rho mapping. Proc Intl Soc Mag Reson Med 2022; (30): 4063.
8. Peng Q, Wu C, Kim J, Li X. Efficient phase-cycling strategy for high-resolution 3D gradient-echo quantitative parameter mapping. NMR Biomed 2022; 35(7): e4700.
Figures