1847
Glycogen Imaging of The Human Liver Using GraspNOE-Dixon1BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Keywords: Liver, Magnetization transfer
We aim to translate the glycogen imaging approach to clinical scanners for human liver imaging. By combining saturation transfer preparation and Golden-angle Radial Sparse Parallel (GRASP) imaging, we can achieve steady-state saturation suitable for generating glycogen nuclear Overhauser enhancement (NOE) contrast and free-breathing imaging readout. Furthermore, we incorporated multi-echo acquisition, enabling NOE quantification in water-only images without fat influence. In this project, we demonstrated the effect of fat signal in z spectra from the composite images and the removal of such effect in z spectra from water only images. We also validated the glycoNOE signal using a fasting protocol.Introduction
Liver glycogen deposits serve as a store of glucose for the use throughout the body. Excess blood glucose triggers insulin release, glycogen synthesis and storage, and during high energy demand or periods of fasting, glucagon signals the liver to break down glycogen and release glucose into circulation to maintain blood glucose homeostasis. The hepatic glycogen metabolism may be altered in diseases such as glycogen storage diseases, liver cirrhosis, type I and type II diabetes as well as in patient who underwent bariatric surgeries. However, in vivo imaging of the glycogen and its metabolism has been challenging.Recently, it was reported that the liver glycogen can be detected in vivo based on its nuclear Overhauser enhancement (GlycoNOE) (1). However, no fat suppression strategies were applied in this initial study which could potentially lead to pseudo NOE signals since the liver contains significant amount of fat. In this project, we aimed to translate the glycoNOE approach to clinical scanners for human liver imaging. By combining saturation transfer preparation and Golden-angle Radial Sparse Parallel (GRASP) imaging (2), we can achieve steady-state saturation suitable for generating glycoNOE contrast and free-breathing liver imaging. Furthermore, we have also incorporated multi-echo acquisition (3) to enable NOE quantification in water-only images without the influence of fat. Lastly, this technique, named GraspNOE-Dixon, was applied to validate NOE signal using a fasting protocol.
Methods
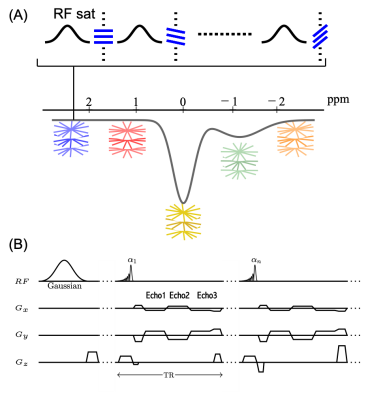
GraspNOE-Dixon sequence: A saturation transfer prepared sequence with stack-of-stars golden-angle radial trajectory as shown in Figure 1. The saturation transfer preparation is performed right before the acquisiton of each radial stack (i.e., all spokes corresponding to one acquisiton angle). There is no delay between acquisitions of different stacks to achieve steady-state saturation transfer imaging. Acquisition of each radial stack is done with centric-out ordering, and the rotation from one stack to the next follows the golden-angle scheme (i.e., adding 111.25°).Imaging experiments: For saturation transfer labeling, each saturation pulse was applied with a duration of 50 ms and a power of 0.7 μT, followed by a short spoiling period (20 mT/m, 1 ms) and acquisition of one radial stack containing 3 echoes. All imaging experiments were performed on a 3T clinical MRI scanner (Skyra, Siemens Healthcare). 16 slices, FOV = 350x350 mm2, in-plane resolution 2.7x2.7 mm2, slice thickness = 5 mm, TR = 5.77 ms, TE1 = 1.41 ms, TE2 = 3.01 ms, TE3= 4.61 ms, and FA= 5°. Two reference images at 390 ppm were collected at the beginning of the dynamic scan and 29 images were acquired within a frequency range of -7.8 to 7.8 ppm. 100 radial stacks were acquired in each dynamic measurement, corresponding to a total scan time of 7 min.
GRASP-Pro reconstruction (4) was performed to reconstruct the dynamic images. The temporal basis needed for subspace construction was generated from the k-space center projections in stack-of-stars k-space. The size of the subspace was set as 10. Fat/water separation was performed after image reconstruction.
Fasting experiment: A healthy individual was scanned and instructed to fast 12 hours before a repeated scan on the second day.
Results and Discussions
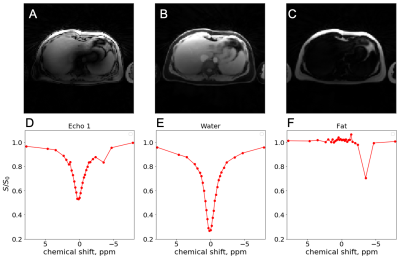
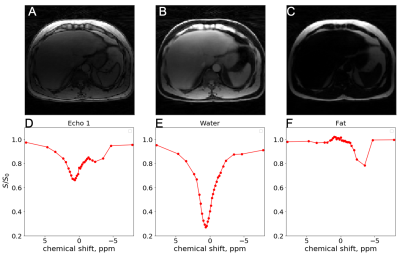
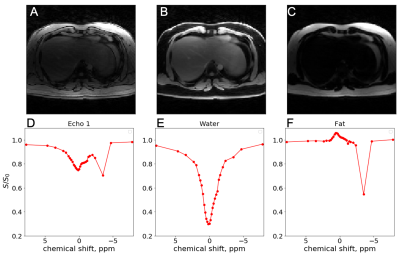
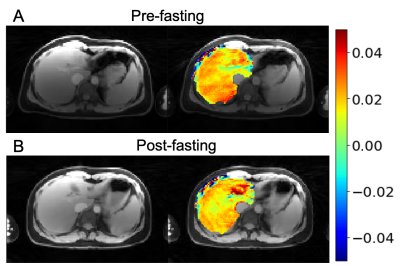
Figures 2,3 and 4 show in-vivo liver images acquired using the GraspNOE-Dixon method, which allows separation of water and fat. Figure 2 shows the composite image from the first echoes, the water image, the fat image and their corresponding saturation transfer spectra (z spectra) of a healthy individual with normal liver fat (3% based on proton density fat fraction (PDFF) imaging). While fat does not participate in exchange transfer, its direct saturation has profound effect on the composite z spectrum, as shown in Figure 2D. Figure 3 shows an individual with elevated liver fat (28% based on PDFF) and figure 4 shows an individual with nonalcoholic fatty liver disease (17%). One can see the z spectra from the composite images was further affected by the fat signal, where the water direct saturation peak was significantly scaled down by the fat contribution. By separating the water and fat signal, the shape of the z spectra was recovered (Figures 2E, 3E and 4E) from the water only images. The residual signal on the negative side of the z spectra may have come from NOE exchange pathways of other mobile proteins (-2 to -5 ppm).GlycoNOE-weighted images were generated by taking the average difference between the acquired water Z-spectra and the Lorentzian fitted (5) spectra between -1 to -2 ppm. Figure 5 shows the NOE maps overlaid on anatomical images before (A) and after (B) fasting. The glycoNOE signal decreased from 2.15% to 1.54% upon fasting.
Conclusion
In this work, we report a clinically applicable imaging protocol called GraspNOE-Dixon, which enables 3D free-breathing multi-echo imaging of glycogen in the human liver at 3T. We demonstrated that fat has a significant effect on the saturation transfer spectra. When fat and water signal are separated, the shape of the saturation transfer spectra can be well recovered from the water only images. Using GraspNOE-Dixon, we found NOE signal decreased upon fasting, which is consistent with a decrease level of glycogen.Acknowledgements
R00EB026312References
1. Zhou Y, van Zijl PCM, Xu X, Xu J, Li Y, Chen L, Yadav NN. Magnetic resonance imaging of glycogen using its magnetic coupling with water. Proceedings of the National Academy of Sciences 2020;117(6):3144-3149.
2. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2014;72(3):707-717.
3. Zhang S, Seiler S, Wang X, Madhuranthakam AJ, Keupp J, Knippa EE, Lenkinski RE, Vinogradov E. CEST-Dixon for human breast lesion characterization at 3 T: A preliminary study. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2018;80(3):895-903.
4. Feng L, Wen Q, Huang C, Tong A, Liu F, Chandarana H. GRASP-Pro: imProving GRASP DCE-MRI through self-calibrating subspace-modeling and contrast phase automation. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2020;83(1):94-108.
5. Jones CK, Huang A, Xu J, Edden RAE, Schär M, Hua J, Oskolkov N, Zacà D, Zhou J, McMahon MT, Pillai JJ, van Zijl PCM. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage 2013;77(0):114-124.
Figures