1845
Ammonium quantification in human plasma by 1H-NMR for staging of liver fibrosis in alcohol and non-alcoholic-related liver disease1MIPMED, IBEC, Barcelona, Spain, 2Liver Unit, Hospital Clínic, Barcelona, Spain, 3Biosensors for Bioengineering, IBEC, Barcelona, Spain, 4IDIBAPS, Barcelona, Spain
Synopsis
Keywords: Liver, Blood, Ammonium quantification, chronic liver disease, hepatic dysfunction, disease biomarker, NMR
Invasive and painful liver biopsy is the gold standard for liver disease diagnosis. Non-invasive methods to assess liver fibrosis in patients with alcohol-related liver disease (ArLD) and non-alcoholic fatty liver disease (NAFLD) are an unmet clinical need. We have developed a robust and reliable 1H NMR protocol to quantify the endogenous ammonium concentration in biological fluids. The measurement of ammonium in blood plasma samples of ArLD and NAFLD patients discerned between some stages of the disease retrospectively and showed that ammonium readout is a robust diagnostic marker of fibrosis stage, more so than current clinically assessed blood hepatic biomarkers.
Introduction
Alcohol-related liver disease (ArLD) and nonalcoholic fatty liver disease (NAFLD) are currently the two most frequent causes of liver disease in the Western world1. The current gold standard for liver disease diagnosis is liver biopsy, which is invasive and may pose significant side effects2. There has been a growing interest in developing noninvasive and clinically acceptable methods to assess the presence of liver fibrosis in patients with ArLD and NAFLD 3. We have developed a method using 1H NMR to quantify endogenous ammonium in blood plasma and shown it to be a sensitive biomarker of liver disease. The ammonium cation (NH4+) is an essential biomolecule in blood derived from the breakdown of nucleic acids and amino acids4. The physiological concentration of NH4+ in blood at equilibrium is about 40 μM in healthy subjects but may be significantly altered due to pathological conditions. In this study we demonstrate that ammonium can be accurately and consistently measured in human blood plasma using a simple protocol to discern between a healthy subject, early stages of liver fibrosis and advanced stages of liver fibrosis.Methods
Each plasma sample (0.4 – 0.6 ml) was transferred into a 2 ml conical flask and treated with TFA (200 μl) to acidify the solution. The sample was snap-frozen in liquid nitrogen and lyophilized for at least 8 hours. The residue was dissolved in a solution containing TFA (200 μl), DMSO (300 μl) and DMSO-d6 (300 μl), and centrifuged (10.000 rpm, 5 min). The liquid phase was removed from the conical flask and the whole sample (800 μl) was transferred into a 5-mm-o.d. NMR tube.NMR spectra of ammonium in aqueous solutions were acquired at 300 K using a 14.1 T Bruker Avance-II+ spectrometer. Subsequent NMR experiments in DMSO were performed at 298K and 9.4 T in a Bruker Avance-III HD spectrometer equipped with a cryoprobe and TopSpin 2.1 software.
Acquisition parameters for all 1H-NMR measurements: acquisition time of 2.5 s; 64 K data points; 90º flip angle; d1 = 15 s; and 128 scans.
Results
The sample treatment protocol was optimized with DMSO-d6 as deuterated solvent and TFA to shift the NH3/NH4+ equilibrium towards the ammonium form (Fig. 1) in order to detect and quantify unequivocally the corresponding triplet peak at 7.1 ppm (Fig. 2).The resulting ammonium concentrations obtained from the samples extracted from healthy subjects (control group) and subjects with initial and advanced stages of either ArLD or NAFLD are shown in Figure 3. The plasma ammonium concentration of healthy subjects was 41.76 ± 1.64 μM. Plasma ammonium concentrations of patients with initial stages of liver disease were not significantly different than those of control samples, regardless of etiology (p = 0.085 for control vs. alcoholic, and p = 0.13 for control vs. nonalcoholic). Plasma ammonium concentration of patients with advanced stages of disease was approximately five times higher than the controls (p < 0.0001 for both). There was no significant difference between groups with alcoholic and nonalcoholic-related initial stages of the disease. Another finding of this study is that ammonium concentration is strongly correlated with platelet levels (p = 0.075) and with INR, especially for the ArLD group (Fig. 4).
Discussion
Previous studies have determined the ammonium concentration in non-biological samples by 1H NMR5,6. However, this has been the first time it has ever been quantified in a biological fluid. In this work we characterized a reliable methodology to accurately diagnose and stage liver fibrosis in alcoholic and non-alcoholic-related liver disease non-invasively. Even though the ammonium quantification cannot fully discern between healthy subjects and early stages, this may be explained by the internal variation of around 50 μM in the early-stage disease diagnosis window, with disease severity varying in each patient. Advanced stages of the disease are easily and significantly discerned from early stages and healthy subjects.Conclusion
In conclusion, we have developed a robust, reliable, and biopsy-free protocol to quantify ammonium in biological fluids with a wide dynamic range of concentrations, relative to other methods currently available. To demonstrate potential applications within medical diagnostics and disease staging, we quantified the ammonium concentration in blood plasma samples of patients suffering from a steatohepatic liver condition and showed that the method can be used to discern between some stages of the disease retrospectively. Prospective studies with a larger cohort of patients should validate the clinical applicability and, in future, the protocol could be used in a clinical setting either for population screening or to assess treatment efficacy.Acknowledgements
This work has been possible thanks to the financial support through the Junior Leader Postdoctoral Fellowship Programme from “la Caixa” Banking Foundation (LCF/BQ/ PI18/11630020), Spanish MINECO project MCIN/AEI/10.13039/501100011033 (Ref. EIN2020-112209), BIST-“la Caixa” Banking Foundation Chemical Biology programme and RYC2020-029099-I, HG received financial support through the FI Fellowship Programme from AGAUR (Ref. 2021 FI_B_01039). the European Union “NextGenerationEU”/PRTR and the European Union's Horizon 2020 (FET Open) under grant agreement GA-863037 (BLOC). The work was also supported by grant PI20/00658 (to JMS) and CIBERDEM from the Instituto de Salud Carlos III.
References
1. Sepanlou S, Safiri S, Bisignano C, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245-266.
2. Role of liver biopsy in nonalcoholic fatty liver disease. Accessed October 1, 2021.
3. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237-264.
4. Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51(3):1062-1069.
5. Hodgetts RY, Kiryutin AS, Nichols P, et al. Refining universal procedures for ammonium. Published online 2020:5-10.
6. Nielander AC, McEnaney JM, Schwalbe JA, et al. A versatile method for ammonia detection in a range of relevant electrolytes via direct nuclear magnetic resonance techniques. ACS Catal. 2019;9(7):5797-5802.
Figures
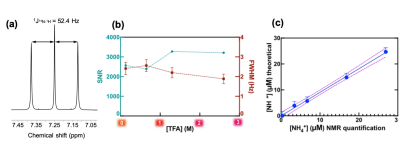
Figure 1: Ammonium chloride in dimethyl sulfoxide and deuterated dimethyl sulfoxide (1:1 DMSO/DMSO-d6). (A) 1H-NMR spectrum. NMR acquisition parameters: 32 scans; 15 s relaxation delay time; 8.5 μs pulse width. (B) Signal-to-noise ratio (SNR) and full width at half maximum (FWHM) as a function of final trifluoroacetic acid (TFA) concentration. (C) [NH4+] calibration curve with 2.6 M TFA added to the DMSO solution.
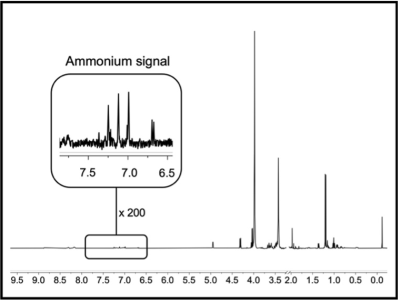
Figure 2: Representative proton nuclear magnetic resonance (1H-NMR) spectrum of blood plasma from an advanced-stage nonalcoholic fatty liver disease patient after lyophilization and dissolving in dimethyl sulfoxide (1:1 DMSO/DMSO-d6) and TFA. NMR acquisition parameters: 128 scans; 15 s relaxation delay time; 8.5 μs rf pulse width.
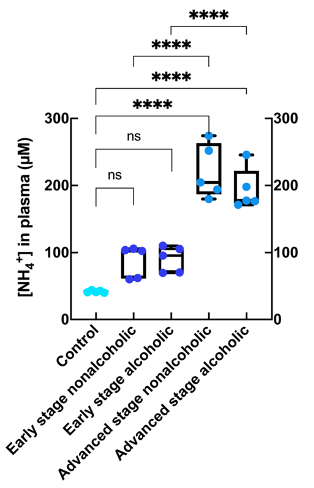
Figure 3: Concentration of ammonium present in plasma from healthy subjects (control; n = 5), initial and advanced stages of alcohol- related fatty liver disease (n = 5 for each group), and initial and advanced stages of nonalcoholic fatty liver disease (n = 5 for each group); **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
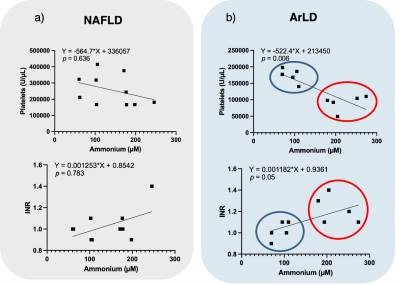
Figure 4: Nonparametric Spearman correlations between platelets and ammonium concentration (top) and international normalized ratio (INR) and ammonium concentration (bottom) for patients with liver disease from either (A) Nonalcoholic fatty liver disease (NAFLD; n = 10) or (B) Alcohol-related liver disease (ArLD; n = 10). The blue and red circles show the two data groups corresponding to initial and advanced stages of alcoholic fatty liver disease.