1840
Fast multi-slice liver water T1 mapping using single-shot continuous inversion recovery spiral imaging1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Philips GmbH Market DACH, Hamburg, Germany, 3Philips Research Lab, Hamburg, Germany
Synopsis
Keywords: Liver, Relaxometry
Liver T1 mapping can characterize liver alterations in diffuse or focal (e.g. metastatic) liver disease, but it is currently limited to acquisitions of a single-slice per breath-hold. We propose a continuous inversion recovery methodology combining a single-shot gradient echo spiral readout, Dixon processing for water-fat separation and dictionary-based analysis for water selective T1 mapping in the liver at 3T. The spiral readout is employed for a high k-space sampling efficiency, enabling acquisitions with only 1.2s per slice. The method allows multi-slice full-liver water T1 mapping either in a single 11s-breath-hold or in a respiratory-triggered acquisition.
Purpose
Liver diseases cause approximately 2 million deaths per year worldwide1. Quantitative MRI is a valuable tool for the characterization of liver diseases. In particular, T1 maps have been applied to differentiate cirrhotic from non-cirrhotic liver2 and to characterize hepatic lesions3.The modified Look-Locker inversion-recovery (MOLLI)4,5 method enables T1 mapping in synchronization with cardiac motion and typically takes one breath hold per slice. MOLLI has been used for abdominal T1 mapping6-8; however, its duration remains a drawback. Rapid continuous inversion-recovery Look-Locker (CIR-LL) methods for multi-slice liver T1 mapping have been proposed employing radial9-10 and spiral11 k-space trajectories. Such approaches typically employ long breath holds (~20s) and map the composite liver signals (water+fat). However, liver T1 mapping is biased in the presence of fat12,13. Dixon14 has been recently applied for rapid water-fat separated liver T1 mapping using radial CIR-LL15.
This work proposes a CIR-LL method combining a spiral readout with Dixon and dictionary-based processing for water selective T1 mapping in the liver. The spiral readout is employed for a high k-space sampling efficiency enabling a multi-slice acquisition in one breath hold at 1.2s per slice or respiratory triggered acquisitions.
Methods
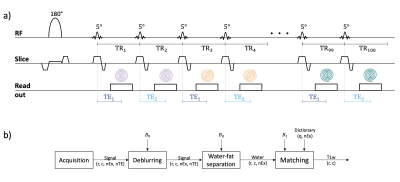
Pulse sequence design: A continuous inversion recovery spoiled gradient echo sequence is employed (Figure 1a). After an adiabatic slice selective inversion, a 5° RF-pulse is repeated 100 times with a TR=12ms. A spiral readout of 5.6ms is acquired at TE1=2.3 or TE2=3.3ms and is rotated every 2 excitations to enable varying spatial encoding. The acquisition lasts 1.2s per slice for 50 excitations per echo. B0 and transmit B1 maps are acquired as pre-scans.Quantification methodology: Using the B0 map, the acquired images are deblurred16 and water-fat separation is performed17. Finally, the water signal is matched to a dictionary to obtain the T1 map of water (T1w). The dictionary simulates the sequence effect on the evolution of signals with T1=[400:5:1300]ms, T2=[40:2:80]ms and B1=[0.55:0.01:1.55]. The B1-correction matching uses the pre-scan B1 map. Figure 1b shows the described pipeline.
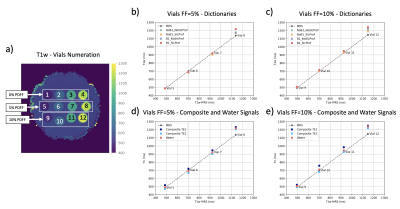
Phantom measurements: To corroborate the T1 range captured by the sequence and the water-fat separation accuracy, the method was evaluated with a phantom with different T1w and proton density fat fraction (PDFF) vials (Calimetrix, Madison, WI, USA) as shown in Figure 2a (FOV=240mm, resolution=3x3x10mm3). Due to the small flip angle and the chosen RF-pulse shape, the effect of slice-profile and B1 correction are expected to be minimal. To verify this, in the 5% and 10% PDFF vials, T1w was calculated with dictionaries that include or exclude the corrections. Furthermore, the T1 of a composite signal varies depending on the echo15 and deviates from the actual T1w. To inspect this, the composite signals of both echoes were matched and compared to T1w. A dictionary with both corrections was used for the latter. As a reference, an IR-prepared Magnetic Resonance Spectroscopy (MRS) STEAM (STEAM TI series) was acquired in the aforementioned vials.
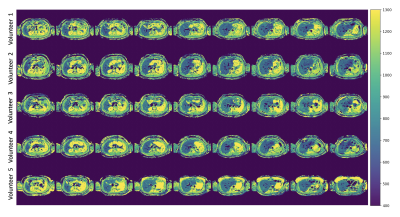
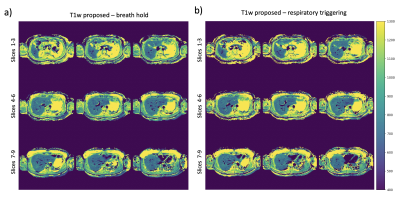
In vivo measurements: A 9-slice acquisition was performed with interleaved slice ordering in 5 volunteers in a single breath hold of 11s (FOV=450mm, resolution=2.81x2.81x10mm3, slice gap=1mm). A second 9-slice acquisition was performed to test repeatability. The dictionary considered B1 and slice-profile effects. MOLLI and MRS were acquired for reference. Due to 1.2s acquisition time per slice, a respiratory triggered scan was performed for the last volunteer eliminating the need of a breath hold.
All experiments were performed at 3T (Philips Elition).
Results
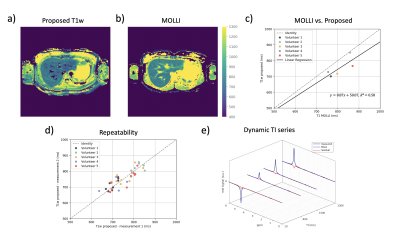
Figures 2b-c show the expected minimal variations of T1w due to slice-profile and B1 correction. The T1w measurements are in agreement with MRS under 1000ms which confirms that 100 excitations can capture the IR-curve of this range, but not higher values as in Vial 8 and Vial 12. Figures 2d-e show T1w in a better agreement with MRS than the T1 of the composite signal at TE1 and TE2 which present variability.Figure 3 shows the in vivo results of all volunteers. The proposed method presents lower values than MOLLI (Figure 4a-b), as the ROI comparison across volunteers also confirms (Figure 4c). The two methods show difference in T1w within vessels. ROIs in different liver slices from two consecutive proposed scans are in agreement (Figure 4e) with an ICC of 0.97. Figure 4e shows the dynamic TI series fitting of one MRS measurement. MRS showed poor repeatability in estimating in vivo T1w; therefore, it is not reported. The short acquisition time per slice made respiratory triggered acquisition possible and comparable to the breath hold scan (Figure 5).
Discussion & Conclusion
The present work proposes a methodology for rapid repeatable multi-slice liver water T1 mapping. The robustness to B1 and slice-profile effects could be further used to save scan and dictionary computation time. The water-fat separation step is particularly relevant for a correct T1 estimation in the presence of fat. T1 of liver is known to be in general under 1000ms18,19; therefore, the presented method is suitable for this application. In contrast, other abdominal organs with much higher T1 may be biased in the T1w maps. The presented 9-slice acquisition fits in a short breath hold of 11s. Due to the 1.2s per slice, the method has shown its applicability for a respiratory triggered acquisition.Acknowledgements
The present research was supported by Philips.References
1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019;70(1):151-171.
2. Kim KA, Park MS, Kim IS, Kiefer B, Chung WS, Kim MJ, Kim KW. Quantitative evaluation of liver cirrhosis using T1 relaxation time with 3 tesla MRI before and after oxygen inhalation. J Magn Reson Imaging 2012;36(2):405-410.
3. Goldberg MA, Hahn PF, Saini S, Cohen MS, Reimer P, Brady TJ, Mueller PR. Value of T1 and T2 relaxation times from echoplanar MR imaging in the characterization of focal hepatic lesions. AJR Am J Roentgenol 1993;160(5):1011-1017.
4. Look DC, Locker DR. Time Saving in Measurement of NMR and EPR Relaxation Times. The Review of Scientific Instruments 1970;41(2):250-251.
5. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52(1):141-146.
6. Cassinotto C, Feldis M, Vergniol J, Mouries A, Cochet H, Lapuyade B, Hocquelet A, Juanola E, Foucher J, Laurent F, De Ledinghen V. MR relaxometry in chronic liver diseases: Comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. Eur J Radiol 2015;84(8):1459-1465.
7. Gilligan LA, Dillman JR, Tkach JA, Xanthakos SA, Gill JK, Trout AT. Magnetic resonance imaging T1 relaxation times for the liver, pancreas and spleen in healthy children at 1.5 and 3 tesla. Pediatr Radiol 2019;49(8):1018-1024.
8. Yoon JH, Lee JM, Paek M, Han JK, Choi BI. Quantitative assessment of hepatic function: modified look-locker inversion recovery (MOLLI) sequence for T1 mapping on Gd-EOB-DTPA-enhanced liver MR imaging. Eur Radiol 2016;26(6):1775-1782.
9. Li Z, Bilgin A, Johnson K, Galons JP, Vedantham S, Martin DR, Altbach MI. Rapid high-resolution T1 mapping using a highly accelerated radial steady-state free-precession technique. J Magn Reson Imaging 2019;49(1):239-252.
10. Goerke U, Ahanonu E, Keerthivasan M, Bilgin A, Deshpande V, Altbach M. Inversion Recovery Look-Locker T1-Mapping for Abdominal Imaging: How Many Slices Can One Fit in a Single Breath-Hold? ISMRM 2022; London, UK.
11. Chen Y, Lee GR, Aandal G, Badve C, Wright KL, Griswold MA, Seiberlich N, Gulani V. Rapid volumetric T1 mapping of the abdomen using three-dimensional through-time spiral GRAPPA. Magn Reson Med 2016;75(4):1457-1465.
12. Mozes FE, Tunnicliffe EM, Pavlides M, Robson MD. Influence of fat on liver T1 measurements using modified Look-Locker inversion recovery (MOLLI) methods at 3T. J Magn Reson Imaging 2016;44(1):105-111.
13. Larmour S, Chow K, Kellman P, Thompson RB. Characterization of T1 bias in skeletal muscle from fat in MOLLI and SASHA pulse sequences: Quantitative fat-fraction imaging with T1 mapping. Magn Reson Med 2017;77(1):237-249.
14. Dixon WT. Simple proton spectroscopic imaging. Radiology 1984;153(1):189-194.
15. Li Z, Mathew M, Syed AB, Feng L, Brunsing R, Pauly JM, Vasanawala SS. Rapid fat-water separated T1 mapping using a single-shot radial inversion-recovery spoiled gradient recalled pulse sequence. NMR Biomed 2022:e4803.
16. Nolte T, Gross-Weege N, Doneva M, Koken P, Elevelt A, Truhn D, Kuhl C, Schulz V. Spiral blurring correction with water-fat separation for magnetic resonance fingerprinting in the breast. Magn Reson Med 2020;83(4):1192-1207.
17. Diefenbach MN, Liu C, Karampinos DC. Generalized parameter estimation in multi-echo gradient-echo-based chemical species separation. Quant Imaging Med Surg 2020;10(3):554-567.
18. Chen Y, Jiang Y, Pahwa S, Ma D, Lu L, Twieg MD, Wright KL, Seiberlich N, Griswold MA, Gulani V. MR Fingerprinting for Rapid Quantitative Abdominal Imaging. Radiology 2016;279(1):278-286.
19. Unal E, Idilman IS, Karcaaltincaba M. Multiparametric or practical quantitative liver MRI: towards millisecond, fat fraction, kilopascal and function era. Expert Rev Gastroenterol Hepatol 2017;11(2):167-182.
Figures